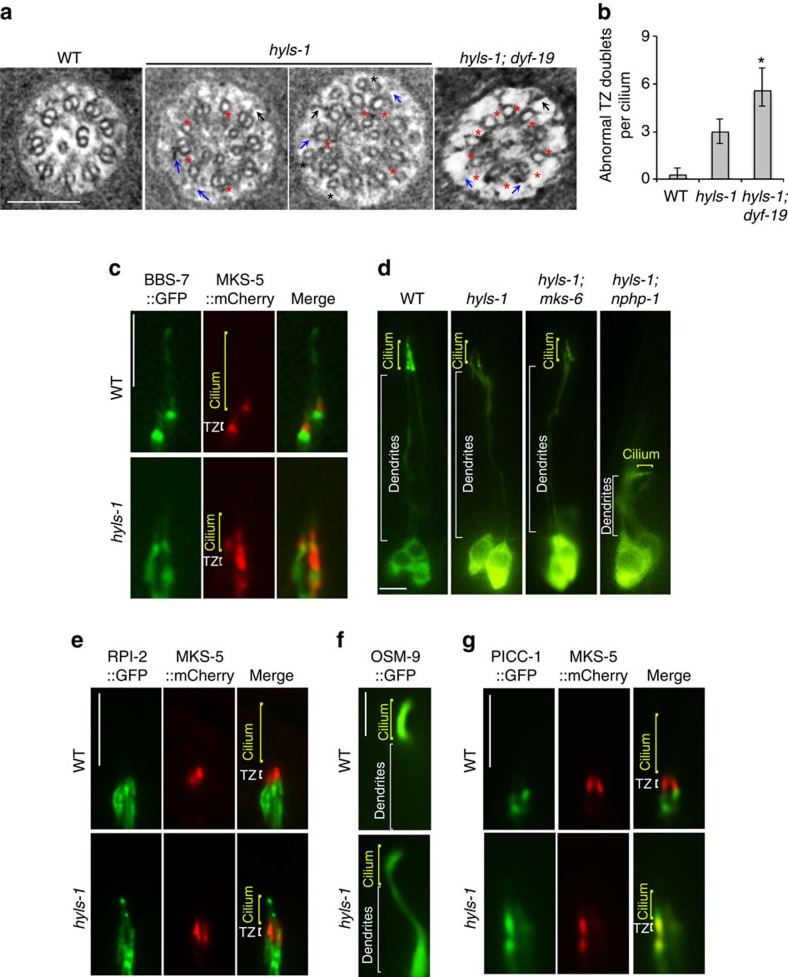
Figure 4. HYLS-1 is required for TZ integrity and function.
(a) TEM analysis of the TZ. hyls-1 mutants display a range of anomalies in the TZ, including missing B-tubules (red stars), putative broken Y-links (blue arrows) and displaced singlet microtubules (black stars). Some Y-links still form and associate with the membrane (black arrows). hyls-1; dyf-19 double mutants display more severe defects with an increased number of incomplete doublet microtubules. (b) Quantification of incomplete doublet microtubules in the TZ in WT, hyls-1 and hyls-1; dyf-19 double mutants. See Supplementary Table 1 for the numbers of cilia analysed for each genetic background. Error bars indicate s.d. Student's t-test for significance; *P<0.01. (c) Fluorescence images of phasmid cilia co-expressing GFP-tagged BBS-7 and mCherry-tagged MKS-5 in WT and hyls-1 mutants. MKS-5 signal exclusively enriches around the TZ in WT cilia, but diffuses to a larger area including the cilia proper in hyls-1 mutants. (d) Dendrite extension is compromised in hyls-1; nphp-1 but not hyls-1; mks-6 double mutants. Representative images of dendrites visualized by expression of GFP-tagged IFT-B component OSM-6 illustrate genetic interaction between HYLS-1 and TZ proteins. See also Supplementary Fig. 4b. (e–g) Ciliary gating for both membrane-associated and soluble proteins is perturbed in hyls-1 mutants. Representative images of phasmid cilia co-expressing GFP-tagged proteins and mCherry-tagged MKS-5 as a TZ marker. (e) The non-ciliary membrane protein RPI-2 is restricted to below the TZ in WT but abnormally enters phasmid cilia in hyls-1 mutants. (f) OSM-9, a membrane receptor, enriches in the cilia of OLQ (outer labial quadrant) neurons in WT worms, but mislocalizes along dendrites in hyls-1 mutants. (g) The non-ciliary cytoplasmic protein PICC-1 is restricted below the TZ in WT but abnormally enters phasmid cilia in hyls-1 mutants. Scale bars, 200 nm (a), 5 μm (c–g).