Abstract
Objective
Pediatric focal low-grade brainstem tumors are associated with excellent prognosis. Surgical resection and conformal radiation therapy are front-line treatment options; radiation therapy (RT) serves as an excellent treatment for disease progression. Given high survival rates and limited research regarding functional outcomes, the current study examined neurocognitive outcomes in a group of low-grade brainstem glioma survivors.
Methods
Forty-three survivors of focal low-grade brainstem gliomas underwent neurocognitive assessment (58% male; median=6.9 years at diagnosis; median=14.9 years at latest assessment). Treatment included combinations of surgery, chemotherapy, and RT with 70% ultimately receiving RT. Neurocognitive outcomes were evaluated through retrospective chart review.
Results
Intellectual and academic performance were significantly different from normative expectations (Full scale IQ=86.5±16.8; Reading Comprehension=91.3±16.4; Math Reasoning=88.2±18.9; reference group=100±15). Further, the percentage performing below average exceeded the expected 16% in the normative sample (Full scale IQ=43%; Reading Comprehension=37%; Math Reasoning=50%). Mean parent ratings did not reflect concerns regarding internalizing and externalizing behaviors or executive functioning (Internalizing=54.9±12.7; Externalizing=51.6±14.6, Global Executive Composite=57.1±16.0; reference group=50±10); however, the proportion with clinically elevated scores was higher than the expected 16% (Internalizing=42%; Externalizing=26%; Global Executive Composite=38%). Mean performance fell below average for visual-motor coordination (81.8±13.2) and parent ratings of adaptive functioning (73.4±24.2), with 65% and 62% falling outside the average range, respectively. There were no significant differences between those receiving and not receiving RT.
Conclusions
Multiple cognitive domains were significantly different from normative expectations. Despite focal disease and treatment targeting subcortical brain regions, neurocognitive risks exist that may impact treatment planning and caregiver education.
Keywords: cognitive, outcomes, brainstem, tumors, childhood
Introduction
Gliomas are the most common brain tumor in children with brainstem gliomas accounting for about 10-20% of all pediatric central nervous system (CNS) tumors [1-3]. Children are typically less than ten years of age at diagnosis and the incidence is similar across genders. Brainstem gliomas are most often (80%) diffuse intrinsic pontine gliomas (DIPG) characterized by short symptom duration prior to diagnosis, cranial nerve involvement, and low survival rates [1]. The remaining 20% are more focal in nature, lower grade, and have excellent prognosis. The 5- and 10-year overall survival rates at our institution have recently been reported as 98% and 90%, respectively [3]. While focal low-grade brainstem gliomas are a heterogeneous group of tumors, they all arise in the brainstem, are most often less than 2 cm in diameter, and are typically classified according to location, size and imaging characteristics. Surgical resection and conformal radiation therapy are front-line treatment options, and radiation therapy (RT) serves as an excellent treatment for disease progression after surgery [3].
It is well established that children who are treated with RT and/or chemotherapy, as well as surgery alone, are at significant risk for cognitive late effects including deficits in intellectual functioning and academic achievement [4-7]. Conformal and intensity modulated radiation therapy, which limit the dose of radiation to the affected brain tissue, have the ability to minimize cognitive risks and better preserve cognitive outcomes [8-9]. Most late effects research has focused on higher-grade tumors, such as medulloblastoma, in part due to the higher rate of toxicities [10]. The more limited literature that examines the overarching population of low-grade gliomas has shown an increased risk across several cognitive domains including intellectual functioning, academic achievement, and adaptive behaviors [10-14]. Cognitive late effects may impact survivors well into adulthood and are associated with lower educational attainment, underemployment, more emotional adjustment problems, and a decreased rate of marriage [1, 15]. Prior literature reviews suggest that survivors of low-grade gliomas are an understudied population that deserves attention and prospective monitoring of cognitive outcomes [10]. Our group previously examined the clinical characteristics, treatment management, and survival rates of patients diagnosed with focal low-grade brainstem gliomas [3]. Given the high survival rates and limited research regarding functional outcomes for this population, we expanded upon this study in order to examine neurocognitive outcomes. We predicted that cognitive functioning would be largely intact given tumor mass effect and the margin of RT mostly excludes areas typically considered responsible for higher cortical functions.
Methods
We reviewed medical and psychological records of 52 patients diagnosed with focal low-grade brainstem gliomas between May 1986 and May 2010. At the time of review, 43 of the 52 (83%) identified patients participated in at least one cognitive assessment. For patients that participated in multiple assessments, we used the most recent assessment for primary analysis. Patients were either assessed at specified time points as part of a treatment protocol (n=20) or were referred for a clinical evaluation by a medical team member (n=23). Assessments that were part of a treatment protocol were administered at specified time intervals during and following the course of treatment. Clinically referred assessments were based on the concerns of the medical team at any given time during and following treatment. Due to the wide range of assessment measures and the time span of our review, we combined measures assessing the same cognitive construct to form the following domains: intellectual functioning, academic achievement, adaptive functioning, visual-motor integration, social-emotional functioning, attention, and executive functioning. All reported measures have large, representative normative standardization samples with demonstrated reliability and validity.
Intellectual functioning, either estimated (EIQ) or full scale intelligence quotient (FSIQ) scores, were obtained primarily from age-appropriate Wechsler measures and also included the Stanford-Binet-V, McCarthy Scales of Children's Abilities, and Reynolds Intellectual Assessment Scales [16-27]. While EIQ is highly correlated with FSIQ [28], EIQ does not include tasks that specifically target working memory and processing speed as are included in the more comprehensive FSIQ. Assessment of academic achievement included reading comprehension and math reasoning subtests from Wechsler, Woodcock Johnson, and Wide Range Achievement measures [29-35]. Adaptive functioning was assessed by parent ratings on either the Vineland Adaptive Behavior Scales or the Adaptive Behavior Assessment System [36-37]. Visual-motor integration was assessed by patient performance on a test of visual-motor coordination, the Beery-Buktenica Developmental Test of Visual-Motor Integration [38]. These measures provide standard scores with a mean of 100 and standard deviation of 15.
Social-emotional functioning was assessed by parent report of internalizing (e.g., anxiety or depression) and externalizing behaviors (e.g., conduct problems or oppositionality); either the Child Behavior Checklist or Behavior Assessment System for Children was given [39-40]. A computerized measure, Conners' Continuous Performance Test, was used to assess sustained attention [41-42]. Parents provided ratings of executive functioning on the Behavior Rating Inventory of Executive Function in the areas of behavioral regulation, metacognition and global executive function [43]. These measures provide T-scores with a mean of 50 and standard deviation of 10.
Overall survival and event-free survival were estimated using the method of Kaplan and Meier [44]. Examination of QQ plots and tests for normality using the Shapiro-Wilk and Kolmogorov-Smirnov statistics were performed. T-tests were used to compare demographic variables for those that received a clinical referral for cognitive assessment and those that completed a standard assessment according to a treatment protocol. Descriptive statistics for cognitive measures were calculated and one sample t-tests were performed in order to compare our sample means to normative means. Chi-square goodness of fit tests were used to compare our sample to what is expected in the normative population, where 16% would be expected to fall one standard deviation outside the average range. Wilcoxon rank sum tests were used to compare neurocognitive outcomes between patients referred for clinical assessments and those that completed standard protocol assessments. We compared cognitive measures of those receiving and not receiving RT using Wilcoxon rank sum tests. Profile plots were used to explore assessments of cognitive outcomes for each patient over time. The effects of RT group and age at diagnosis on the change in neurocognitive outcomes was examined for the measures with adequate sample size using random coefficients models.
The study was approved by the institutional review board at St. Jude Children's Research Hospital.
Results
Ten year estimates of overall survival and event-free survival were 92% (standard error, 5%) and 54% (standard error, 10%), respectively. Table 1 shows clinical and treatment characteristics. Among the 43 patients with cognitive assessments, 58% were male and 79% were Caucasian. The median age at diagnosis was 6.9 years (range 0.7 – 17.3 years), median age at most recent assessment was 14.9 years (range 6.6 – 23.3 years), and median number of years from diagnosis at the most recent assessment was 6.1 (range 0.01 – 12.9). The median number of cognitive evaluations per patient was 5 (range 1 – 14). The most common tumor type was pilocytic astrocytoma (67%). Treatment consisted of surgical resection, RT, chemotherapy, or a combination of these modalities. Upfront treatment was most often surgery only (42%), followed by RT (28%), with a total of 70% of patients ultimately receiving RT during the course of treatment. The median dose of RT was 54 Gy (range 52.2 – 70.2 Gy) and duration of RT ranged from 20 to 46 days. Additional clinical characteristics of this sample are published [3]. There were no significant differences with respect to gender (p=0.54), age at diagnosis (p=0.73), age at last assessment (p=0.37) or EIQ (p=0.17) between those who completed assessments due to clinical referral or protocol based evaluation.
Table 1. Patient Clinical and Treatment Characteristics.
| N (%) | |
|---|---|
|
| |
| Gender | |
| Male | 25 (58%) |
| Female | 18 (42%) |
|
| |
| Pathology | |
| Pilocytic astrocytoma | 29 (67%) |
| Astrocytoma | 10 (23%) |
| Pilomyxoid | 2 (5%) |
| Ganglioma | 1 (2%) |
| Oligodendroglioma | 1 (2%) |
|
| |
| Appearance | |
| Intrinsic | 20 (47%) |
| Exophytic | 23 (53%) |
|
| |
| Anatomic Location | |
| Medulla | 14 (33%) |
| Midbrain | 17 (40%) |
| Pons | 12 (28%) |
|
| |
| Race | |
| White | 34 (79%) |
| Black | 6 (14%) |
| Black & White | 1 (2%) |
| Other | 1 (2%) |
| Unknown | 1 (2%) |
|
| |
| Treatment Era | |
| 1985-1996 | 20 (47%) |
| 1997-2010 | 23 (53%) |
|
| |
| Upfront Primary Treatment | |
| Chemo only | 1 (2%) |
| Chemo+RT | 1 (2%) |
| RT only | 12 (28%) |
| Surgery only | 18 (42%) |
| Surgery+RT | 7 (16%) |
| Surgery+Chemo | 3 (7%) |
| Surgery+Chemo+RT | 1 (2%) |
|
| |
| RT (upfront or at progression) | |
| Yes | 30 (70%) |
| No | 13 (30%) |
|
| |
| Type of RT (n=30) | |
| Two-dimensional radiotherapy | 10 (33%) |
| Three-dimensional radiotherapy | 19 (63%) |
| Intensity modulated radiotherapy | 1 (3%) |
|
| |
| Maximal Surgery Extent before any Adjuvant Treatment | |
| Gross total resection | 8 (19%) |
| Near total resection | 13 (30%) |
| Subtotal resection | 8 (19%) |
| Biopsy | 14 (33%) |
Upfront Primary Treatment=Initial treatment; RT=Radiation Therapy; Chemo=Chemotherapy; Surgery=Surgical resection
Table 2 shows descriptive statistics for all cognitive measures at the most recent assessment. The mean scores for FSIQ (p=.0002), reading comprehension (p=.0066), math reasoning (p=.0019), adaptive functioning (p=.0019), and visual-motor integration (p<.0001) were significantly different than respective normative means. Given adaptive functioning measures are more often given to patients with lower intellectual functioning, to establish a diagnosis of Intellectual Disability [45], EIQ was compared post hoc between those who did and did not receive adaptive functioning questionnaires (88.4±23.9 vs. 99.0±21.0; p=.11) to evaluate potential administration bias. EIQ, social-emotional functioning, attention, and executive functioning domains were not significantly different than respective normative means at p<.01. When we examined all domains further, we found that larger proportions of the sample had scores outside the average range (20-65%) compared to what is expected in the normative population (16%). Fig 1 Proportions of Scores Outside Average Range shows the domains in which proportions of scores differed significantly (p<.05) from expectations.
Table 2. Cognitive Outcomes at the Most Recent Assessment.
| Measures with Standard Scores (M=100; SD=15) | N | Mean ± SD | P-value* | N (%) Below Average | P-value** |
|---|---|---|---|---|---|
| Intellectual Functioning | |||||
| Estimated IQa | 33 | 94.6 ± 22.6 | 0.1767 | 12 (36%) | 0.0014 |
| Full Scale IQ | 28 | 86.5 ± 16.8 | 0.0002 | 12 (43%) | 0.0014 |
| Academic Achievement | |||||
| Reading Comprehension | 30 | 91.3 ± 16.4 | 0.0066 | 11 (37%) | 0.0020 |
| Math Reasoning | 30 | 88.2 ± 18.9 | 0.0019 | 15 (50%) | <0.0001 |
| Adaptive Functioning | |||||
| Adaptive Functioning Composite | 13 | 73.4 ± 24.2 | 0.0019 | 8 (62%) | <0.0001 |
| Visual Motor Integration | |||||
| Beery VMI | 17 | 81.8 ± 13.2 | <0.0001 | 11 (65%) | <0.0001 |
|
| |||||
| Measures with T-scores (M=50; SD=10) | N | Mean ± SD | P-value* | N (%) Clinically Elevated | P-value* |
|
| |||||
| Social-Emotional Functioning | |||||
| Internalizing behaviors | 31 | 54.9 ± 12.7 | 0.0411 | 13 (42%) | <0.0001 |
| Externalizing behaviors | 31 | 51.6 ± 14.6 | 0.5435 | 8 (26%) | 0.1364 |
| Executive Functioning | |||||
| Behavioral Regulation | 13 | 55.9 ± 17.4 | 0.2490 | 5 (38%) | 0.0272 |
| Metacognition | 13 | 57.2 ± 15.1 | 0.1101 | 4 (31%) | 0.1463 |
| Global Executive Function | 13 | 57.1 ± 16.0 | 0.1361 | 5 (38%) | 0.0272 |
| Attention | |||||
| Commissions | 15 | 53.9 ± 8.8 | 0.1111 | 4 (27%) | 0.2598 |
| Hit Reaction Time | 15 | 48.1 ± 11.7 | 0.5453 | 3 (20%) | 0.6726 |
| Detectability | 14 | 56.7 ± 8.3 | 0.0101 | 6 (43%) | 0.0061 |
| Response Style | 14 | 65.4 ± 21.5 | 0.0191 | 6 (43%) | 0.0061 |
We calculated Estimated IQ scores for the assessments in which the Full Scale IQ was obtained and the appropriate subtests were given for Estimated IQ scores. In addition, some assessments contained only Estimated IQ scores
P-values were obtained by t-tests comparing sample means with a normative mean of 100 and SD of 15 for standard scores and a mean of 50 and SD of 10 for T-scores
P-values were obtained using Chi-square goodness of fit tests, where in a normative population 16% would be expected to have scores <85 (standard scores) or >60 (T-scores)
Figure 1. Proportions of Scores Outside Average Range.
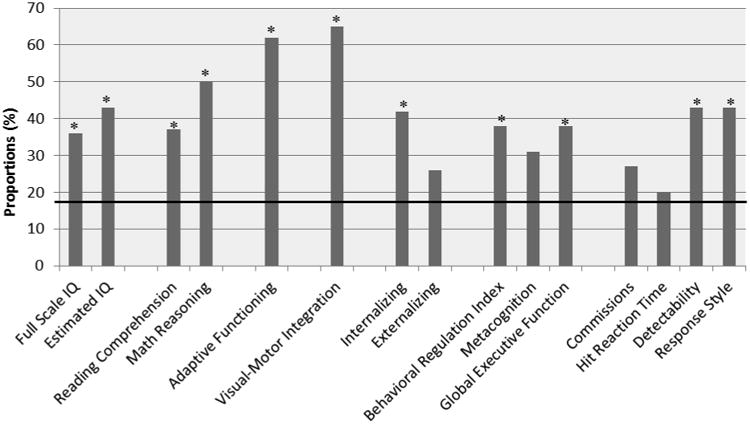
* Proportion of scores significantly different (1SD) than expected 16% in normative population
▬ Indicates 16% expected in normative sample
When comparisons were made between the patients that received clinically referred assessments and those that completed standard protocol assessments, there were no significant differences across cognitive domains (p=.14-.99). There was no significant difference across cognitive domains between those receiving RT (n=28) and not receiving RT (n=15), (p=.07-0.73). When examining the data there was no evidence of a significant effect of age at diagnosis on cognitive outcomes with adequate data for model inclusion (EIQ, FSIQ, reading comprehension, math reasoning, internalizing and externalizing behaviors; p<.019). There was also no evidence of a significant change in IQ over time (ESIQ p=.29; FSIQ p =.44) and no evidence of an effect of receiving RT on ESIQ or FSIQ (p=.32; p=.43, respectively).
Discussion
As a group, measures of FSIQ, academic achievement, adaptive functioning, and visual-motor coordination were significantly lower than normative means. Additionally, there was an excess of below average scores when compared to the normative population. The percentage of survivors falling one standard deviation outside the average range (indicative of worse performance) was significantly higher across most cognitive domains than the percentage we expect to find in a sample of healthy peers. Furthermore, patients who received assessments completed as a clinical referral did not significantly differ on any of the cognitive outcomes when compared to those who received a standard protocol evaluation, suggesting a representative sample.
Adaptive functioning indices fell below the average range; however, parent report of adaptive functioning for this sample may not be representative due to a potential measurement selection bias. Clinically, the measures used to assess adaptive functioning are more often given to patients with lower intellectual functioning, as impairment in adaptive functioning is a requirement for establishing a diagnosis of Intellectual Disability [45]. Accordingly, there was a trend for average EIQ to be higher for those who did not receive adaptive functioning questionnaires. Although the difference was not statistically significant, it could represent a domain assessed only when its deficit is anticipated and not an area that is below average across the entire sample. Group scores for visual-motor coordination also fell below average; however, this is to be expected in a sample that may have cranial nerve and motor impairment. This is consistent with a higher group mean for EIQ than FSIQ given the latter is more dependent on speeded psychomotor functioning.
An aim of this study was to compare cognitive outcomes among patients who did and did not receive RT. At the time of the most recent assessment, 28 patients had received RT. There was no significant difference between those receiving and not receiving RT across cognitive domains at the most recent assessment. We found no significant effects of RT or changes across time on measures of intellectual functioning. However, it must be considered that these analyses were conducted with a small sample and we were further limited by the specific measures given per patient resulting in some cognitive domains with limited data when compared to others. Also, our small sample size limited our ability to make dose-specific RT comparisons. Furthermore, we were unable to tease apart the effect of the tumor in comparison to effect of treatment due to the retrospective nature of this study. Additional limitations inherent to retrospective studies include the length of the time covered by the review, in order to capture enough patients and evaluate change over time, and the variety of measures utilized across evaluations.
Current findings are in line with expectations based on the existing literature. It is well established that survivors of childhood brain tumors are at significant risk for cognitive late effects. Research has focused on examining more malignant/higher grade brain tumors, such as childhood medulloblastoma, primarily due to lower grade tumors typically having a benign course, less toxic treatments, and better recovery. This study adds to the existing literature by revealing that cognitive risk may not be as prominent or intensive as higher grade tumors but does, in fact, exist.
These risks are present despite focal disease and treatment focused on subcortical brain structures. These findings are consistent with well-established treatment-related white matter injury that can disrupt efficient subcortical-cortical transmission [46]. Damage to specific neural pathways connected to higher cortical regions has shown an impact on specific cognitive late effects in brain tumor survivors [47]. Further, pediatric patients treated with surgery only for low-grade tumors, including brainstem tumors, are at risk for neurocognitive late effects presumably due to disruptions to neuroconnectivity including cerebellar-cortical pathways [12]. Thus, cognitive deficits identified in this study can result from direct tumor and surgical effects such as visual-motor and processing speed deficits secondary to encroachment of the pyramidal tracts, indirect tumor and surgical effects such as attention and executive dysfunction due to disruption of subcortical-cortical transmission, or late effects of adjuvant therapy such as deficits in attention, executive function and processing speed due to white matter changes associated with adjuvant therapies. Core deficits in attention and executive functions can subsequently affect rate of learning such that declines are found over time in global indices such as intellectual, academic and adaptive functioning. The clinical implications of these findings are concerning when considering the overall quality of life of these survivors, specifically with performance in academic and employment settings. The social burden of life-long accrued disabilities and under-employment may be substantial and something to be addressed in order to improve patient care.
Low-grade gliomas arising in the brainstem are a heterogeneous group of tumors that are understudied. Current findings suggest that this population is at risk and importance should be placed on neurocognitive monitoring. As survival rates increase, the need for identifying ways to increase quality of life becomes of paramount importance. Future directions include prospective, longitudinal trials (with neurocognitive monitoring beginning at diagnosis) in order to identify risk factors and specific areas of cognitive decline, as well as assessment of more distal functional outcomes such as quality of life and achievement of milestones including college graduation, employment, and independent living. It can be concluded that although this group has focal disease and received targeted treatments in subcortical brain regions, neurocognitive risks still exist. Considering the high survival rates, these findings may play a role in treatment planning, education of caregivers regarding treatment alternatives, and cognitive interventions.
References
- 1.Minturn JE, Fisher MJ. Gliomas in children. Curr Treat Option N. 2013;15:316–327. doi: 10.1007/s11940-013-0225-x. [DOI] [PubMed] [Google Scholar]
- 2.Recinos PF, Sciubba DM, Jallo GI. Brainstem tumors: Where are we today? Pediatr Neurosurg. 2007;43:192–201. doi: 10.1159/000098831. [DOI] [PubMed] [Google Scholar]
- 3.Klimo P, Pai Panandiker AS, Thompson CJ, Frederick AB, Qaddoumi I, Gajjar A, Armstrong GT, Ellison DW, Kun LE, Ogg RJ, Sanford RA. Management and outcome of focal low-grade brainstem tumors in pediatric patients: The St. Jude experience. J Neuros-Pediatr. 2013;11:274–281. doi: 10.3171/2012.11.PEDS12317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ris MD, Noll RB. Long-term neurobehavioral outcome in pediatric brain-tumor patients: review and methodological critique. J Clin Exp Neuropsyc. 1994;16:21–42. doi: 10.1080/01688639408402615. doi:10.1080/ [DOI] [PubMed] [Google Scholar]
- 5.Moore BD. Neurocognitive outcomes in survivors of childhood cancer. J Pediatr Psychol. 2005;30:51–63. doi: 10.1093/jpepsy/jsi016. [DOI] [PubMed] [Google Scholar]
- 6.Merchant TE, Fouladi M. Ependymoma: new therapeutic approaches including radiation and chemotherapy. J Neuro-Oncol. 2005;75:287–299. doi: 10.1007/s11060-005-6753-9. [DOI] [PubMed] [Google Scholar]
- 7.Mulhern RK, Merchant TE, Gajjar A, Reddick WE, Kun LE. Late neurocognitive sequelae in survivors of brain tumours in childhood. Lancet Oncol. 2004;5:399–408. doi: 10.1016/S1470-2045(04)01507-4. [DOI] [PubMed] [Google Scholar]
- 8.Merchant TE, Mulhern RK, Krasin MJ, Kun LE, Williams T, Li C, Xiong X, Khan RB, Lustig RH, Boop FA, Sanford RA. Preliminary results from a phase II trial of conformal radiation therapy and evaluation of radiation-related CNS effects for pediatric patients with localized ependymoma. J Clin Oncol. 2004;22:3156–3162. doi: 10.1200/JCO.2004.11.142. [DOI] [PubMed] [Google Scholar]
- 9.Conklin HM, Ashford JM, Howarth RA, Merchant TE, Ogg RJ, Santana V, Reddick WE, Wu S, Xiong X. Working memory performance among childhood brain tumor survivors. Journal of International Neuropsychological Society. 2012;18:996–1005. doi: 10.1017/S1355617712000793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ris MD, Beebe DW. Neurodevelopmental outcomes of children with low-grade gliomas. Dev Disabil Res Rev. 2008;14:196–202. doi: 10.1002/ddrr.27. [DOI] [PubMed] [Google Scholar]
- 11.Beebe DW, Ris MD, Armstrong FD, Fontanesi J, Mulhern R, Holmes E, Wisoff JH. Cognitive and adaptive outcome in low-grade pediatric cerebellar astrocytomas: Evidence of diminished cognitive and adaptive functioning in national collaborative research studies (CCG 9891/POG 9130) J Clin Oncol. 2005;23:5198–5204. doi: 10.1200/JCO.2005.06.117. [DOI] [PubMed] [Google Scholar]
- 12.Ris MD, Beebe DW, Armstrong D, Fontanesi J, Holmes E, Sanford RA, Wisoff JH. Cognitive and adaptive outcome in extracerebellar low-grade brain tumors in children: A report from the Children's Oncology Group. J Clin Oncol. 2008;26:4765–4770. doi: 10.1200/JCO.2008.17.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Armstrong GT, Conklin HM, Huang S, Srivastava D, Sanford R, Ellison DW, Merchant TE, Hudson MM, Hoehn ME, Robison L, Gajjar A, Morris B. Survival and long-term health and cognitive outcomes after low grade glioma. J Neuro-Oncol. 2011;13:223–234. doi: 10.1093/neuonc/noq178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Merchant TE, Conklin HM, Wu S, Lustig RH, Xiong X. Late effects of conformal radiation therapy for pediatric patients with low-grade glioma: prospective evaluation of cognitive, endocrine, and hearing deficits. J Clin Oncol. 2009;27:3691–3697. doi: 10.1200/JCO.2008.21.2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gurney JG, Krull KR, Kadan-Lottick N, Nicholson S, Nathan PC, Zebrack B, Tersak JM, Ness KK. Social outcomes in the childhood cancer survivor study cohort. J Clin. 2009;27:2390–2395. doi: 10.1200/JCO.2008.21.1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wechsler D. Wechsler abbreviated scale of intelligence. Harcourt Assessment; San Antonio: 1999. [Google Scholar]
- 17.Wechsler D. Manual for the Wechsler intelligence scale for children, revised. Psychological Corporation; New York: 1974. [Google Scholar]
- 18.Wechsler D. Wechsler intelligence scale for children. 3rd. Psychological Corporation; San Antonio: 1991. [Google Scholar]
- 19.Wechsler D. Wechsler intelligence scale for children. 4th. Pearson; San Antonio: 2003. [Google Scholar]
- 20.Wechsler D. Wechsler adult intelligence scale, revised. Psychological Corporation; San Antonio: 1981. [Google Scholar]
- 21.Wechsler D. Wechsler adult intelligence scale. 3rd. Harcourt Assessment; San Antonio: 1999. [Google Scholar]
- 22.Wechsler D. Wechsler adult intelligence scale. 4th. Pearson; San Antonio: 2008. [Google Scholar]
- 23.Roid G. Stanford-Binet intelligence scales. 5th. Riverside Publishing; Rolling Meadows: 2005. [Google Scholar]
- 24.Andrews JW. Test review of reynolds intellectual assessment scales. J Psychoeduc Assess. 2007;25:402–408. doi: 10.1177/0734282907300381. [DOI] [Google Scholar]
- 25.Wechsler D. Wechsler preschool and primary scale of intelligence, revised. The Psychological Corporation; San Antonio: 1989. [Google Scholar]
- 26.Wechsler D. Wechsler preschool and primary scale of intelligence. 3rd. The Psychological Corporation; San Antonio: 2002. [Google Scholar]
- 27.McCarthy D. McCarthy scales of children's abilities. The Psychological Corporation; New York: 1972. [Google Scholar]
- 28.Sattler J. Assessment of children. 3rd. Jerome M Sattler, Publisher Inc; San Diego: 1992. [Google Scholar]
- 29.Woodcock R, McGraw K, Mather N. Woodcock-Johnson 3rd edn, tests of achievement. Riverside Publishing; Itasca: 2001b. [Google Scholar]
- 30.The Psychological Corporation. Wechsler individual achievement test. Harcourt, Brace, Jovanovich, Inc; New York: 1992. [Google Scholar]
- 31.The Psychological Corporation. Wechsler individual achievement test screener. The Psychological Corporation; San Antonio: 1992. [Google Scholar]
- 32.Wechsler D. Wechsler individual achievement test. 2nd. The Psychological Corporation; London: 2005. [Google Scholar]
- 33.Wechsler D. Wechsler individual achievement test. 3rd. Harcourt, Brace, Jovanovich, Inc; New York: 1992. [Google Scholar]
- 34.Jastak S, Wilkinson GS. Wide range achievement test-revised administration manual. Jastak Associates; Wilmington: 1984. [Google Scholar]
- 35.Wilkinson GS. The wide range achievement test: manual. 3rd. Wide Range, Inc; Wilmington: 1993. [Google Scholar]
- 36.Sparrow SS, Balla DA, Cicchetti DV. Vineland adaptive behavior scales. American Guidance Service; Circle Pines: 1984. [Google Scholar]
- 37.Harrison PL, Oakland T. Adaptive behavior assessment system. 2nd. The Psych Corp; San Antonio: 2003. [Google Scholar]
- 38.Beery KE. The beery-buktenica developmental test of visual-motor integration: administration, scoring and teaching manual. 4th. Modern Curriculum Press; Parsippany: 1997. [Google Scholar]
- 39.Achenbach TM. Manual for the CBCL. University of Vermont; Burlington: 1991. [Google Scholar]
- 40.Reynolds CR, Kamphaus RW. Behavior assessment system for children. 2nd. Pearson Assessments Resources, Incorporated; Bloomington: 2004. [Google Scholar]
- 41.Conners CK. Conners' continuous performance test II. Multi-Health Systems, Inc; North Tonawanda: 2000. [Google Scholar]
- 42.Conners CK. Conners' continuous performance test. Multi-Health Systems, Inc; North Tonawanda: 1995. [Google Scholar]
- 43.Gioia GA, Isquith PK, Guy SC, Kenworthy L. Behavior rating inventory of executive function. Psychological Assessment Resources Inc; Odessa: 2000. [Google Scholar]
- 44.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. doi: 10.2307/2281868. [DOI] [Google Scholar]
- 45.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th. American Psychiatric Association; Washington: 2013. [Google Scholar]
- 46.Reddick WE, White HA, Glass JO, Wheeler GC, Thompson SJ, Gajjar A, Leigh L, Mulhern RK. Developmental model relating white matter volume to neurocognitive deficits in pediatric brain tumor survivors. Cancer. 2003;97:2512–2519. doi: 10.1002/cncr.11355. [DOI] [PubMed] [Google Scholar]
- 47.Law N, Bouffet E, Laughlin S, Laperriere N, Briere M, Strother D, McConnell D, Hukin J, Fryer C, Rockel C, Dickson J, Mabbott D. Cerebello-thalamo-cerebral connections in pediatric brain tumor patients: impact on working memory. NeuroImage. 2011;56:2238–2248. doi: 10.1016/j.neuroimage.2011.03.065. [DOI] [PubMed] [Google Scholar]


