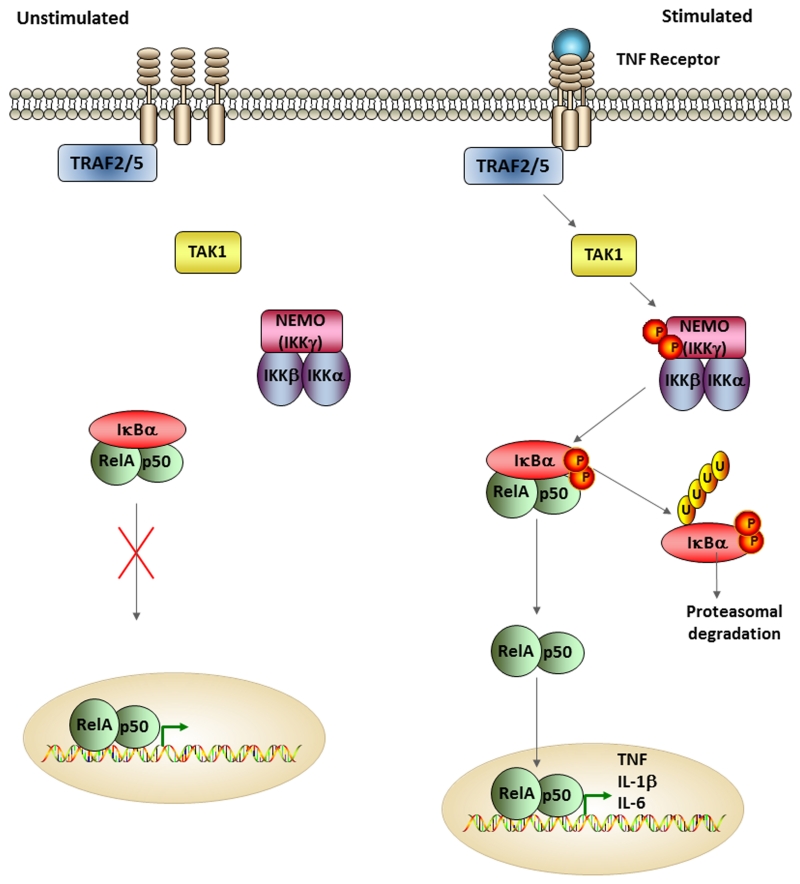
Figure 1. The Canonical NF-κB Signaling Pathway.
This schematic demonstrates some of the major steps associated with the canonical NF-κB signaling pathway under both unstimulated and stimulated conditions. The canonical pathway is triggered by a variety of stimuli that activate diverse receptors, such pattern recognition receptors, TNF receptors, and proinflammatory cytokine receptors. In this representative image, the TNF receptor is shown. When unstimulated, the IKK complex composed of NEMO (IKKγ), IKKβ, and IKKα, along with the heterodimer composed of NF-κB proteins RelA and p50 are inactive and located in the cytoplasm. The binding of a ligand to the cell surface receptor, such as TNF binding to TNF receptor, leads to the recruitment of adaptor proteins, such as TRAF2 or TRAF5 and TAK1. This upstream activity leads to the phosphorylation and activation of the regulatory subunit of the IKK complex, NEMO, which in turn leads to the phosphorylation of the catalytic subunit of the IKK complex, IKKβ. IKKβ then mediates the phosphorylation and induction of proteosomal degradation of IκBα, which then allows for nuclear localization of the heterodimer RelA/p50. Nuclear localization leads to the transcription of proinflammatory cytokines such as TNF, IL-1β, and IL-6.