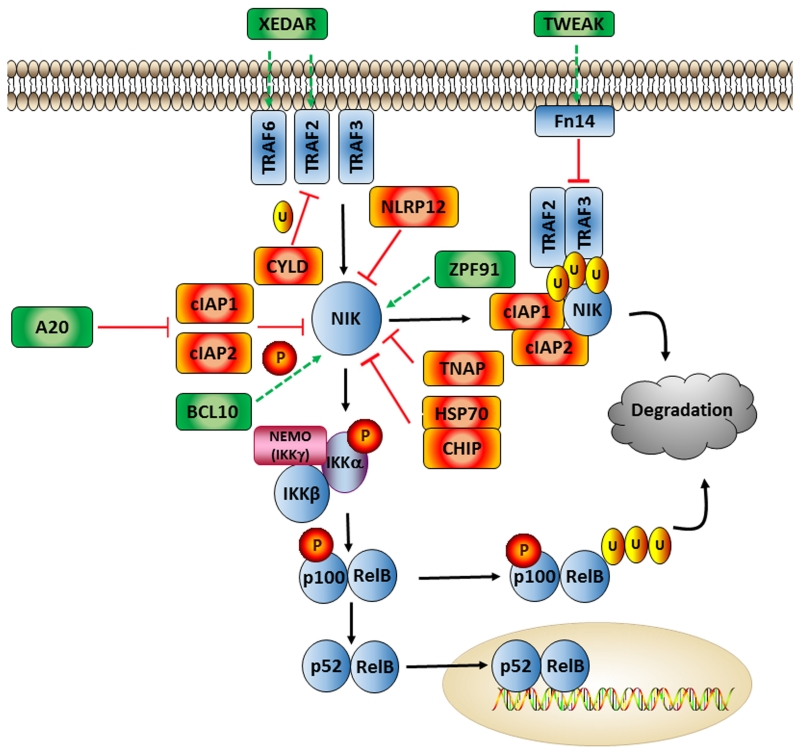
Figure 3. Major Positive and Negative Regulators of Non-canonical NF-κB Signaling.
Much of the regulation of the non-canonical NF-κB pathway revolves around stabilization or degradation of NIK. cIAP1/cIAP2 are the most well-known suppressors of NIK activity, forming a complex with TRAF2 and TRAF3, resulting in ubiquitination and proteosomal degradation. NLRP12 performs a similar function by binding to NIK and enhancing the formation of this complex. HSP70 also downregulates NIK via its partner CHIP, and TNAP interacts with NIK, TRAF2, and TRAF3 and inhibits NIK’s kinase activity. CYLD negatively regulates non-canonical NF-κB signaling further upstream, by ubiquinating TRAF2 and therefore inhibiting CD40 and XEDAR signaling. In terms of activators, XEDAR activates non-canonical NF-κB signaling and stimulates NIK by binding to TRAF6 and TRAF2. Also at the receptor level, TWEAK/Fn14 binding disassociates the ubiquitination complex itself and frees NIK. A20 binds to cIAP1/2 and also prevents proper NIK degradation. BCL10 controls the phosphorylation of NIK and promotes its activity, while ZPF91 binds directly to NIK to augment function.