Abstract
Long-term exposure to organophosphate pesticides (OPs) without acute poisoning can lead to various OPs. Environmental exposure to organophosphate pesticides may be associated with depression and suicide attempts in a population living in a rural agricultural area. Patients (n = 149) suffering from major depressive disorder (with and without attempted suicide) and a control group of healthy individuals (n = 64) who had been living in the same rural district for at least 1 year were selected. Red blood cell acetylcholine esterase (RBC-AChE) activity was examined as the basis of evaluating the degree of chronic environmental exposure to OPs residues. There were negative association between RBC-AChE activity levels and suicide attempts, the number of past suicide attempts and hopelessness levels in the depressive patients. The results of the study may support the idea that environmental exposure to OPs may be associated with mental health in individuals living in agricultural districts who are not farmers or working in occupations with access to OPs.
Keywords: Acetylcholinesterase, Organophosphate, Depression, Suicide
Introduction
The suicidal behavior as one of the significant public health problem represents the 10th common cause of death in worldwide [1]. Besides the great differences for suicide rates among different countries, some studies have reported the different suicide rates among different regions of the same country as well [2]. High suicide rates have been reported especially in the rural areas [2, 3]. The underlying causes of these regional disparities are not clear. Suicide is a complex phenomenon and may be the result of an interaction of multiple biological, psychological and socioeconomic factors as well as environmental pollutants [4]. High suicide rates were also reported in areas where organophosphates were used [5].
Organophosphates (OPs) are the most widely used group of pesticides and the use and availability of them are significant concerns for the studies on mental health. Not only these chemicals are used for suicide attempts but also their possession may be directly associated with mental disorders [6]. Pesticides exposure is reported as a possible risk factor for Parkinson’s disease, Alzheimer’s disease, attention deficit hyperactivity disorder, depressive and anxiety disorders and for mortality ascribed to mental disorders [7–9]. The association between OPs storage and suicidal ideation has been reported for several years [6].
Although OPs are widely used in agriculture and even household environments as insecticides, their usage is not sufficiently controlled, and then poisoning has become a significant public health issue [10]. OP poisoning by inhalation, swallowing and through contact with the eyes or skin are grouped in three main categories; occupational accidents, intentional poisoning (as attempted suicide), and non-occupation-related accidental poisoning (in the household) [4]. Toxic effects of low-dose, long-term environmental exposure to OPs without acute poisoning are matters for researches recently. The consumption of foods contaminated with OPs residues is the most frequent reason for exposure [11]. Two of the other causes of low dose-long term exposure to pesticides are related to the consumption of contaminated water and the inhalation of contaminated air by residues [12].
The family of cholinesterases (ChEs) is one of the most important enzymes in the bodies of human beings, other vertebrates and insects and necessary for the health of the central nervous system. The mechanism of action of OPs pesticides involves the accumulation of acetylcholine in the nervous system through the inhibition of acetylcholinesterase (AChE). There are three types of AChE enzymes in human beings: Red blood cell (erythrocytic) AChE (AChE), plasma ChE (pseudocholinesterase), and cerebral AChE [13]. AChE in red blood cells and nervous system are the similar types of enzyme [14]. Plasma ChE is synthesized in the liver. The activity levels of AChE and pseudocholinesterase can be measured in the blood. Plasma ChE and AChE activities have been used for monitoring workers who were exposed to OPs and also used for investigating accidental exposures. Firstly, plasma ChE activity is immediately inhibited after exposure, and this is used to evaluate the OPs poisoning at an early stage, while AChE is used to evaluate long-term exposure. Plasma enzyme activity shows an exponential pattern of recovery. Because its half-life time is 12 days, recovery is completed in 50 days and is consistent with the de novo synthesis rate. The mean recovery of AChE activity appears linear over time. The enzyme returns to its unexposed activity after about 82 days; this depends on the life-span of red blood cells [15]. For these reasons chronic exposure to OPs in human beings has been correlated with AChE activity [16].
According to our knowledge, there isn’t enough research subjecting the psychological effects of environmental exposure to OPs among a non-farmer population living in an agricultural district and this study tries to propound those effects for OPs. This study was designed to examine the relation between AChE activity levels and environmental low-dose, long term exposure to OPs in a population living in an agricultural area who had suicidal behavior with depression.
Materials and methods
Study population and design
The present study was carried out in the Suleyman Demirel University Medical Faculty Research and Clinical Practices Hospital, in Isparta, Turkey. Subjects were enrolled to study from inpatient, outpatient and emergency departments of psychiatry clinic. The SCID-1 (Structured Clinical Interview for DSM-IV Axis I Disorders) was applied for the psychiatric diagnosis of the patients and controls [17]. Beck’s Depression Inventory [18], Beck’s Anxiety Inventory [19], Impulsivity Scale [20] and Hopelessness Scale [21] were used to evaluate depression, anxiety, hopelessness and impulsivity levels of patients and healthy controls.
The patients diagnosed as major depressive disorder (MDD, totally n = 149) were divided into two groups according to the subjects who had attempted suicide (n = 73) or not (n = 76). A healthy control group (n = 64) was selected from the general population and hospital personnel. The patients selected for the study, as well as the healthy controls, were chosen from the local community and who had been living in the Isparta-Burdur agricultural district for at least 1 year.
Suicide methods are commonly distincted into two main categories. Firearm, hanging, cutting with sharp objects, jumping from high places and corrosive substance drinking are classified as violent methods; while overdose and poison by gases are classified as nonviolent methods.
Subjects who had attempted suicide with organophosphates, either on this occasion or previously, and diagnosed with MDD related to organic etiologies, alcohol or substance addiction, farmers, or persons working at jobs where OPs are used were excluded from the study. It had been postulated that the second and third generation cholinesterase inhibitors clinically used in the treatment of Alzheimer’s Disease (Tacrine, Donepezil, rivastigmine, Galanthamine) inhibit AChE in a therapeutic efficacy window at a rate of 30–60 % [22]. For this reason patients taking medications to treat Alzheimer’s disease were excluded from the study. Subjects with anemia were also excluded from the study.
Ethical considerations
The study protocol was approved by the Suleyman Demirel University Medical Faculty Board of Ethics. Informed consent was obtained from all individual participants included in the study.
Determination of AChE activity
After obtaining the informed consent 4 ml of venous blood was drawn from each participant into tubes containing K2EDTA to determine the level of AChE activity The anticoagulated blood was separated into plasma and erythrocytes by centrifugation at 1500g for 10 min at +4 °C. The RBC samples were washed three times in cold isotonic saline (0.9 %, v/w) and then hemolyzed with 2 ml of bidistillated water. All samples were preserved at −80 °C until the date of analysis. Hemoglobin concentration was determined by the cyanmethemoglobin method from the hemolyzed erythrocytes [23]. Two biochemists who were blinded to patients and controls measured AChE activity using the colorimetric method defined by Ellman et al. [24]. The amount of substrate hydrolyzed was correlated with hemoglobin concentration levels to calculate AChE activity in terms of International Units/Hemoglobin (IU/HgB).
Statistical analysis
SPSS statistical software package 13 (SPSS; Chicago, IL) was used for all statistical evaluations. Comparisons between groups were analyzed using the Student’s t test, the Mann–Whitney U test, the Chi-square test and ANOVA depending upon the distribution of the variable. Correlation analysis was performed with the Spearman correlation test. ROC analysis was performed to find the cut-off value for the AChE activity (≤316.8443) of patients. Binary logistic regression analysis was made for evaluation of different variables on suicide. We used the Cochran–Mantel–Haenszel (CMH) method to generate an odds ratio adjusted for occupation. Statistical significance was accepted as p < 0.05.
Results
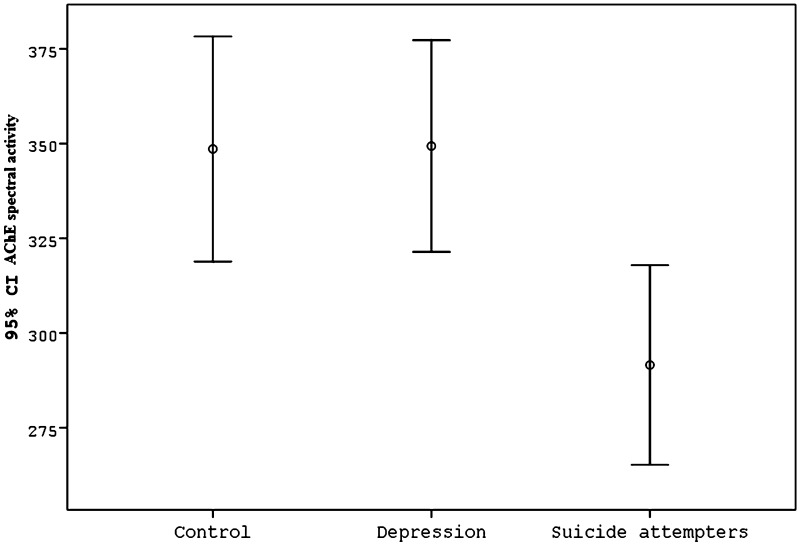
The demographic data, AChE activity for the MDD (with and without suicide attempt) and control groups were presented in Table 1 and methods of attempted suicide were presented in Table 2. There was no significant difference between groups in terms of age, gender, or the area in which the person had lived in the last year (provincial center/surrounding towns and villages). Years of education was lower in the MDD group than in the controls, and unemployed subjects had a higher frequency in the MDD group than in the controls (p < 0.0001). Although AChE activity in the MDD group (321.03 ± 120.00 IU/HgB) was lower than in the healthy control group (348.58 ± 118.94 IU/HgB), the difference was not significant (p = 0.127). When the MDD group was divided into those who had attempted suicide and those who had not, it was found that levels of AChE activity of the MDD patients who had attempted suicide (n = 73) (291.56 ± 112.94 IU/HgB) were significantly lower than those of MDD patients who had not attempted suicide (n = 76) (349.34 ± 122.21 IU/HgB), and those of the healthy controls (348.58 ± 118.94 IU/HgB) (p = 0.004) (Fig. 1). In logistic regression models, the unadjusted odds ratio (OR) for the association between AChE activity and suicide attempt was 0.392 (95 % confidence interval, CI 0.209–0.734 and p = 0.003). Years of education, gender and age were not significant but occupation was significantly associated with suicide attempt in this model. After the adjustment for occupations in the logistic regression analysis, the OR for the association between AChE activity and suicide attempt was ORCMH = 0.394 (95 % confidence interval, CI 0.214–0.727 and p = 0.003).
Table 1.
Demographic characteristics and AChE activity of MDD and controls
| Variables | Controls (n = 64) | MDD (n = 149) | |
|---|---|---|---|
| Age (years, interval) | 25.5 (22.3–31) | 27 (22–39) | p = 0.250 |
| Sex (M/F, %) | 45.3/54.7 | 36.9/63.1 | p = 0.144 |
| Duration of education | 13 (11–16) | 11 (5–13) | p < 0.001 |
| Occupation | p < 0.001 | ||
| Unemployed | 1 | 16 | |
| Housewife | 1 | 43 | |
| Employed | 47 | 66 | |
| Student | 13 | 19 | |
| Retired | 2 | 5 | |
| Place of residence in last 1 year | p = 0.067 | ||
| Provincial center | 56 | 123 | |
| Surrounding village | 8 | 26 | |
| AChE activity (IU/HgB) | 348.58 ± 118.94 | 321.03 ± 120.00 | p = 0.127 |
Table 2.
Suicide attempt types (n: 73)
| n | % | |
|---|---|---|
| Non violent | 55 | 75.3 |
| Overdose | 51 | 69.8 |
| Poison by gases | 4 | 5.5 |
| Violent | 18 | 24.7 |
| Corrosive substance drinking | 4 | 5.5 |
| Cutting with sharp objects | 6 | 8.2 |
| Hanging | 3 | 4.1 |
| Jumping from high places | 4 | 5.5 |
| Firearm | 1 | 1.4 |
Fig. 1.
Comparison of levels of AChE activity of the control 348.58 ± 118.94 IU/HgB, depression (without suicide) 349.34 ± 122.21 IU/HgB and suicide attempters (depression with suicide) 291.56 ± 112.94 IU/HgB (F = 5.672, df = 2, p = 0.004)
A negative correlation was found between the number of suicide attempts of the patients in the past and their AChE activity levels (r = −0.227, p = 0.001).When the patients’ suicide attempt methods were classified as violent (n = 18, 24.7 %) and non-violent (n = 55, 75.3 %) (Table 2), it was seen that the AChE activity levels (256.34 ± 94.73 IU/HgB) of those who had chosen violent methods in their suicide attempts were lower than the levels of patients choosing non-violent suicide methods (303.08 ± 116.76 IU/Hg) but difference was not statistically significant (p = 0.096).
A negative correlation was found between hopelessness levels in the patient group (with and without suicide) and AChE activity levels (r = −0.155, p = 0.025). No correlation was found between patient group AChE activity levels and the Beck depression scale (p = 0.189), the Beck anxiety scale (p = 0.767) and the Impulsiveness scale (p = 0.258).
Discussion
The present study found a strong independent association between low AChE activity levels and suicide attempts, the number of past suicide attempts and hopelessness levels in the depressive patients among a non-farmer population living in an agricultural district. With respect to confounding factors, the logistic regression analysis showed that none of the covariates accounted substantially for the association identified between exposure to OPs and suicide attempts. Long-term exposure to OPs without the existence of acute poisoning has been associated with memory or emotional problems and with neurobehavioral effects such as depression, suicidal thinking, fatigue, alcohol intolerance and reduced performance described as a syndrome named as chronic organophosphate-induced neuropsychiatric disorder (COPIND) in farmers and people who work in occupations with access to OPs [25]. The findings on persons who do not work with OPs as a primary occupation but live in rural districts widely polluted with OPs are very scarce. In communities where pesticides were used and stored; high levels of urine metabolites were determined in children [26]; and the association of urinary OPs metabolites and attention deficit hyperactivity disorder was found also [9]. In addition low AChE activity has been associated with deficits in neurodevelopment, particularly in attention, inhibitory control and memory [27].
The levels of AChE activity of the MDD patients who had not attempted suicide were lower than those of the healthy controls but the difference was not significant. However a negative correlation was found between low AChE activity and hopelessness level in the MDD patients (with and without suicide). In this condition hopelessness may be more associated with suicide than depression [28]. Also we did not find any association between the AChE activity and the impulsivity level of patients, in contrast to studies which reported a relationship between OPs exposure and impulsivity in rats [29]. The variation in impulsivity and depression levels may be caused by OPs exposure dose dependently; this could be established by studies on larger populations.
In our study no significant association between low AChE activity and gender was found, but gender interaction was reported with OPs exposure in several studies [27, 30, 31]. Low AChE activity was related with deficits in neurodevelopment, particularly in attention, inhibition, and memory in boys but not in girls [27]. Prenatally organophosphate exposed children had greater decreases in attention among boys versus girls in agricultural areas in California [30] and in working memory among children in New York City [31]. Animal studies have also determined greater detrimental neurochemical and behavioral effects among males after exposure to organophosphates [32].
Although no special biomarker is determined for detecting long term low-dose exposure to OPs, AChE activity has been correlated with measureable physiologic changes in people exposed to organophosphates [16, 33]. Synaptic and erythrocytic AChE are functionally similar so changes in erythrocytic AChE activity may reflect the situation in target tissues, especially in the brain. OPs has been reported to inhibit brain AChE activity in rats with significant AChE inhibition in the hippocampus [32]. Hippocampus is a structure with important roles in depression, suicidal events, memory and imagination [34]. Animal studies demonstrate that OPs inhibit AChE levels in the brain and produce such effects as keeping serotonin concentration levels low and targeting various neurochemical factors such as growth factors, various neurotransmitters and secondary messengers [35, 36]. Nevertheless, AChE inhibition is a sign of exposure to OPs, other neurotoxic mechanisms may exist also.
Our findings provide insight on AChE inhibition and its short-term association with suicide attempt and hopelessness in depressed patients. Whether patient’s suicidal thinking and hopelessness would improve after full recovery of their AChE activity is not known. Exposure to OPs may be in various routes and may be chronic. OPs can remain active for long periods by reaching soil and water sources and may induce a long-term, low-dose exposure especially in persons living in areas of the intensive OPs use [4]. OP residues have been detected in a variety of fruits and vegetables, in milk, soil, drinking water, fish, and stored grains. Moreover, in several studies from Turkey and from other countries a significant proportion of the samples was determined to contain OPs above the tolerance limit [11, 37].
Our study has certain limitations. One of them is OPs residues in the urine of subjects which could not be measured simultaneously. However short half-lives of OPs in blood (approximately 1 day) and variations in daily and seasonal exposure lead to great daily variability of OPs metabolites in urine [38, 39]. Measurement of AChE activity may overcome these problems. Because AChE inhibition is a physiologic reaction to OPs exposure and AChE levels is not affected by OPs metabolism and individual’s sensitivity. Erythrocytic and neuronal AChE has similar activity and brain AChE has been reported to be derogated after chlorpyrifos exposure in rats [14, 32]. And also erythrocytic AChE has a long salvage time (82 days) [15], intraindividual changeability is low [40], is taken into account as a stable appraisal of past exposures to cholinesterase inhibitors [41]. In epidemiologic studies it is reported that a single measure of AChE activity can be an adequate indicator of pesticide exposure [42]. Second limitation is occupation difference in suicide attempters. Unemployed persons had the lowest AChE activity among the suicide attempters, but we were not able to explain this issue. Other limitation is eating habits and drinking water source. We tried to create similar groups according to socio-demographic and habitat circumstances however we did not include eating habits and drinking water. No measurement could be performed to determine whether food and drinking water contained residues of OPs or not.
In conclusion, the results of the study are consistent with the view that patients with major depression who attempt suicide have been exposed to OPs for longer periods and the possibility of long-term and chronic OPs exposure may be one of the determinants of the attempting suicide. Our study may have significance in terms of pointing to the indirect effects of OPs on the mental health of human beings. Agricultural areas may have a higher level of OPs residues in the soil, drinking water, and air than other regions without agriculture. The local non-farming population may be at risk of mental disorder and especially suicidal behavior. Moreover there may be variations in drug responses during the treatment of suicide attempters due to OPs exposure presence. This is an issue that needs to be investigated with further research.
Compliance with Ethical Standards
Conflict of interest
The authors declare that there is no conflict of interest.
Contributor Information
Vesile Altinyazar, Email: valtinyazar@adu.edu.tr.
Fevziye Burcu Sirin, Phone: 90 232 243 43 43, Email: fbsirin@gmail.com.
Recep Sutcu, Email: rsutcu@hotmail.com.
Ibrahim Eren, Email: drieren@yahoo.com.
Imran Kurt Omurlu, Email: imran.omurlu@adu.edu.tr.
References
- 1.World Health Organization . Figures and facts about suicide. Geneva: World Health Organization; 1999. [Google Scholar]
- 2.Jagodic HK, Agius M, Pregelj P. Inter-regional variations in suicide rates. Psychiatr Danub. 2012;24(1):82–85. [PubMed] [Google Scholar]
- 3.Handley TE, Inder KJ, Kelly BJ, Attia JR, Kay-Lambkin FJ. Urban–rural influences on suicidality: gaps in the existing literature and recommendations for future research. Aust J Rural Health. 2011;19(6):279–283. doi: 10.1111/j.1440-1584.2011.01235.x. [DOI] [PubMed] [Google Scholar]
- 4.London L, Flisher AJ, Wesseling C, Mergler D, Kromhout H. Suicide and exposure to organophosphate insecticides: cause or effect? Am J Ind Med. 2005;47(4):308–321. doi: 10.1002/ajim.20147. [DOI] [PubMed] [Google Scholar]
- 5.Parron T, Hernandez AF, Villanueva E. Increased risk of suicide with exposure to pesticides in an intensive agricultural area: a 12-year retrospective study. Forensic Sci Int. 1996;79(1):53–63. doi: 10.1016/0379-0738(96)01895-6. [DOI] [PubMed] [Google Scholar]
- 6.Zhang J, Stewart R, Phillips M, Shi Q, Prince M. Pesticide exposure and suicidal ideation in rural communities in Zhejiang province, China. Bull World Health Org. 2009;87(10):745–753. doi: 10.2471/BLT.08.054122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ross SM, McManus IC, Harrison V, Mason O. Neurobehavioral problems following low-level exposure to organophosphate pesticides: a systematic and meta-analytic review. Crit Rev Toxicol. 2013;43(1):21–44. doi: 10.3109/10408444.2012.738645. [DOI] [PubMed] [Google Scholar]
- 8.Baltazar MT, Dinis-Oliveira RJ, de Lour des Bastos M, Tsatsakis AM, Duarte JA, Carvalho F. Pesticides exposure as etiological factors of Parkinson’s disease and other neurodegenerative diseases—a mechanistic approach. Toxicol Lett. 2014;230(2):85–103. doi: 10.1016/j.toxlet.2014.01.039. [DOI] [PubMed] [Google Scholar]
- 9.Bouchard MF, Bellinger DC, Wright RO, Weisskopf MG. Attention-deficit/hyperactivity disorder and urinary metabolites of organophosphate pesticides. Pediatrics. 2010;125(6):1270–1277. doi: 10.1542/peds.2009-3058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Konradsen F, van der Hoek W, Cole DC, Huchinson G, Daisley H, Singh S, et al. Reducing acute poisoning in developing countries–options for restricting the availability of pesticides. Toxicology. 2003;192(2–3):249–261. doi: 10.1016/S0300-483X(03)00339-1. [DOI] [PubMed] [Google Scholar]
- 11.Ye M, Beach J, Martin JW, Senthilselvan A. Associations between dietary factors and urinary concentrations of organophosphate and pyrethroid metabolites in a Canadian general population. Int J Hyg Environ Health. 2015;218(7):616–626. doi: 10.1016/j.ijheh.2015.06.006. [DOI] [PubMed] [Google Scholar]
- 12.Cohen Hubal EA, Sheldon LS, Burke JM, McCurdy TR, Berry MR, Rigas ML, et al. Children’s exposure assessment: a review of factors influencing Children’s exposure, and the data available to characterize and assess that exposure. Environ Health Perspect. 2000;108(6):475–486. doi: 10.1289/ehp.00108475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Holmstedt B. Pharmacology of organophosphorus cholinesterase inhibitors. Pharmacol Rev. 1959;11:567–688. [PubMed] [Google Scholar]
- 14.Aaron CK. Organophosphates and carbamates. In: Haddad LMSM, Borron SW, Burns MJ, editors. Haddad and Winchester’s clinical management of poisoning and drug overdose. 4. Philadelphia: Saunders Elsevier; 2007. [Google Scholar]
- 15.Mason HJ. The recovery of plasma cholinesterase and RBC acetylcholinesterase activity in workers after over-exposure to dichlorvos. Occup Med (Lond) 2000;50(5):343–347. doi: 10.1093/occmed/50.5.343. [DOI] [PubMed] [Google Scholar]
- 16.Dasgupta S, Meisner C, Wheeler D, Xuyen K, Thi Lam N. Pesticide poisoning of farm workers-implications of blood test results from Vietnam. Int J Hyg Environ Health. 2007;210(2):121–132. doi: 10.1016/j.ijheh.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 17.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured clinical interview for DSM-IV clinical version. Washington DC: American Psychiatric Press; 1997. [Google Scholar]
- 18.Schotte CK, Maes M, Cluydts R, De Doncker D, Cosyns P. Construct validity of the Beck Depression Inventory in a depressive population. J Affect Disord. 1997;46(2):115–125. doi: 10.1016/S0165-0327(97)00094-3. [DOI] [PubMed] [Google Scholar]
- 19.Ulusoy M, Şahin NH, Erkmen H. Turkish version of the Beck Anxiety Inventory: sychometric properties. J Cogn Psychother. 1998;12(2):163–172. [Google Scholar]
- 20.Apter A, Plutchik R, van Praaq HM. Anxiety, impulsivity and depressed mood in relation to suicidal and violent behavior. Acta Psychiatr Scand. 1993;87(1):1–5. doi: 10.1111/j.1600-0447.1993.tb03321.x. [DOI] [PubMed] [Google Scholar]
- 21.Durak A, Palabıyıkoglu R. Beck Umutsuzluk Ölçeği geçerlik çalışması. Kriz Dergisi. 1994;2(2):311–319. [Google Scholar]
- 22.Benzi G, Moretti A. Is there a rationale for the use of acetylcholinesterase inhibitors in the therapy of Alzheimer’s disease? Eur J Pharmacol. 1998;346(1):1–13. doi: 10.1016/S0014-2999(98)00093-4. [DOI] [PubMed] [Google Scholar]
- 23.Van Kampfen EJ, Zjilstra WG. Determination of hemoglobin and its derivatives. Adv Clin Chem. 1965;8:141–187. doi: 10.1016/S0065-2423(08)60414-X. [DOI] [PubMed] [Google Scholar]
- 24.Ellman GL, Courtney KD, Andres V, Jr, Feather-Stone RM. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol. 1961;7:88–95. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- 25.Ahmed GM, Davies DR. Chronic organophosphate exposure: towards the definition of a neuropsychiatric syndrome. J Nutr Environ Med. 1997;7:169–176. doi: 10.1080/13590849762583. [DOI] [Google Scholar]
- 26.Lambert WE, Lasarev M, Muniz J, Scherer J, Rothlein J, Santana J, et al. Variation in organophosphate pesticide metabolites in urine of children living in agricultural communities. Environ Health Perspect. 2005;113(4):504–508. doi: 10.1289/ehp.6890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Suarez-Lopez JR, Himes JH, Jacobs DR, Jr, Alexander BH, Gunnar MR. Acetylcholinesterase activity and neurodevelopment in boys and girls. Pediatrics. 2013;132(6):1649–1658. doi: 10.1542/peds.2013-0108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Large M, Smith G, Sharma S, Nielssen O, Singh SP. Systematic review and meta-analysis of the clinical factors associated with the suicide of psychiatric in-patients. Acta Psychiatr Scand. 2011;124(1):18–29. doi: 10.1111/j.1600-0447.2010.01672.x. [DOI] [PubMed] [Google Scholar]
- 29.Cardona D, López-Crespo G, Sánchez-Amate MC, Flores P, Sánchez-Santed F. Impulsivity as long-term sequelae after chlorpyrifos intoxication: time course and individual differences. Neurotox Res. 2011;19(1):128–137. doi: 10.1007/s12640-009-9149-3. [DOI] [PubMed] [Google Scholar]
- 30.Marks AR, Harley K, Bradman A, Kogut K, Barr DB, Johnson C, et al. Organophosphate pesticide exposure and attention in young Mexican–American children: the CHAMACOS study. Environ Health Perspect. 2010;118(12):1768–1774. doi: 10.1289/ehp.1002056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Horton MK, Kahn LG, Perera F, Barr DB, Rauh V. Does the home environment and the sex of the child modify the adverse effects of prenatal exposure to chlorpyrifos on child working memory? Neurotoxicol Teratol. 2012;34(5):534–541. doi: 10.1016/j.ntt.2012.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Johnson FO, Chambers JE, Nail CA, Givaruangsawat S, Carr RL. Developmental chlorpyrifos and methyl parathion exposure alters radial-arm maze performance in juvenile and adult rats. Toxicol Sci. 2009;109(1):132–142. doi: 10.1093/toxsci/kfp053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Suarez-Lopez JR, Jacobs DR, Jr, Himes JH, Alexander BH. Acetylcholinesterase activity, cohabitation with floricultural workers, and blood pressure in Ecuadorian children. Environ Health Perspect. 2013;121(5):619–624. doi: 10.1289/ehp.1205431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li M, Long C, Yang L. Hippocampal–prefrontal circuit and disrupted functional connectivity in psychiatric and neurodegenerative disorders. Biomed Res Int. 2015;2015:810548. doi: 10.1155/2015/810548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Burns CJ, McIntosh LJ, Mink PJ, Jurek AM, Li AA. Pesticide exposure and neurodevelopmental outcomes: review of the epidemiologic and animal studies. J Toxicol Environ Health B Crit Rev. 2013;16(6):395–398. doi: 10.1080/10937404.2013.841529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Terry AV., Jr Functional consequences of repeated organophosphate exposure: potential non-cholinergic mechanisms. Pharmacol Ther. 2012;134(3):355–365. doi: 10.1016/j.pharmthera.2012.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Isleyen M, Sevim P, White JC. Accumulation of weathered p, p’-DDTs in grafted watermelon. J Agric Food Chem. 2012;60(4):1113–1121. doi: 10.1021/jf204150s. [DOI] [PubMed] [Google Scholar]
- 38.Bouchard M, Gosselin NH, Brunet RC, Samuel O, Dumoulin MJ, Carrier G. A toxicokinetic model of malathion and its metabolites as a tool to assess human exposure and risk through measurements of urinary biomarkers. Toxicol Sci. 2003;73(1):182–194. doi: 10.1093/toxsci/kfg061. [DOI] [PubMed] [Google Scholar]
- 39.Bradman A, Kogut K, Eisen EA, Jewell NP, Quirós-Alcalá L, Castorina R, et al. Variability of organophosphorous pesticide metabolite levels in spot and 24-hr urine samples collected from young children during 1 week. Environ Health Perspect. 2013;121(1):118–124. doi: 10.1289/ehp.1104808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lefkowitz LJ, Kupina JM, Hirth NL, Henry RM, Noland GY, Barbee JY, Jr, et al. Intraindividual stability of human erythrocyte cholinesterase activity. Clin Chem. 2007;53(7):1358–1363. doi: 10.1373/clinchem.2006.085258. [DOI] [PubMed] [Google Scholar]
- 41.Barr DB, Angerer J. Potential uses of biomonitoring data: a case study using the organophosphorus pesticides chlorpyrifos and malathion. Environ Health Perspect. 2006;114(11):1763–1769. doi: 10.1289/ehp.9062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Suarez-Lopez JR, Jacobs DR, Jr, Himes JH, Alexander BH, Lazovich D, Gunnar M. Lower acetylcholinesterase activity among children living with flower plantation workers. Environ Res. 2012;114:53–59. doi: 10.1016/j.envres.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]