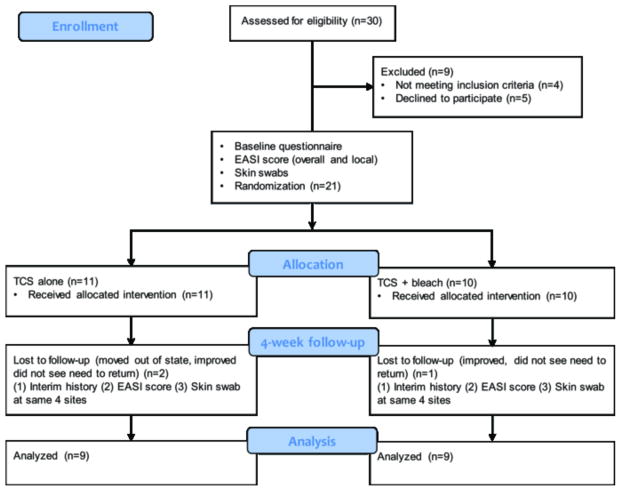
Figure 1. Study Design.
Twenty-one subjects with AD and 14 controls were enrolled. Moderate to severe AD was defined as an investigator’s global assessment (IGA) score of 3 or 4 (0–4 scale). At baseline, a detailed questionnaire was administered, local and overall Eczema Area and Severity Index (EASI) scores were recorded and skin swabs were obtained from 4 clinical sites (3 lesional and 1 nonlesional) in AD patients and at 4 sites of AD predilection in control patients. Ten AD subjects were randomized to receive a bottle of bleach to be diluted into bath water twice weekly to achieve a 0.005% sodium hypochlorite concentration, while 11 (TCS alone group) received an identical bottle containing water and the same instructions for its use. At 4-week follow-up, parents were asked about treatment use, and local and overall EASI scores were again recorded and the 4 same sites were swabbed.