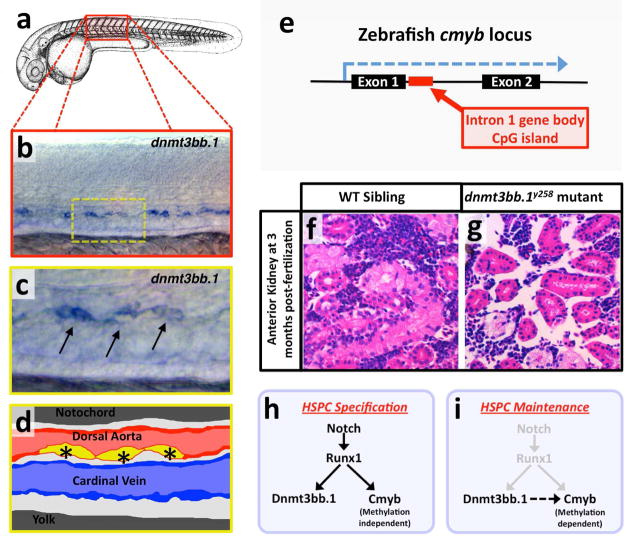
Figure 2. Dnmt3bb.1 is an essential epigenetic factor in HSC fate maintenance.
a, Camera lucida drawing of a zebrafish embryo with a red box noting the approximate region of the trunk shown in the in situ hybridization image in panel b. b,c Whole mount in situ hybridization of zebrafish trunk probed for dnmt3bb.1, showing expression in the developing HSCs (arrows in panel c). Yellow inset box in panel b indicates the magnified area shown in panel c. d, diagram corresponding to panel c showing dorsal aorta (red), cardinal vein (blue), and dnmt-positive HSCs in the floor of the dorsal aorta (yellow, with asterisks). e, Schematic showing the zebrafish cmyb locus and the position of the intron 1 gene body CpG island. f,g, Images through the anterior kidneys (adult hematopoietic organ in zebrafish equivalent to the bone marrow in higher vertebrates) of wild type sibling (f) and dnmt3bb.1y258 mutant (g) adult zebrafish, stained with hematoxylin/eosin, revealing a profound loss of hematopoietic tissue in mutant animals. h, Notch-Runx1 signaling controls specification of HSPCs in the ventral wall of the dorsal aorta during early embryogenesis. This signaling pathway initiates HSPC expression of both the key transcription factor Cmyb and the epigenetic regulator Dnmt3bb.1. i, As development proceeds Runx1 expression is down-regulated and continued maintenance of active Cmyb expression in HSPC depends on Dnmt3bb.1–mediated Cmyb gene body DNA methylation, ensuring maintenance of HSPC cell fate during the Runx1-independent phase. Images are from Gore et al. (2016).