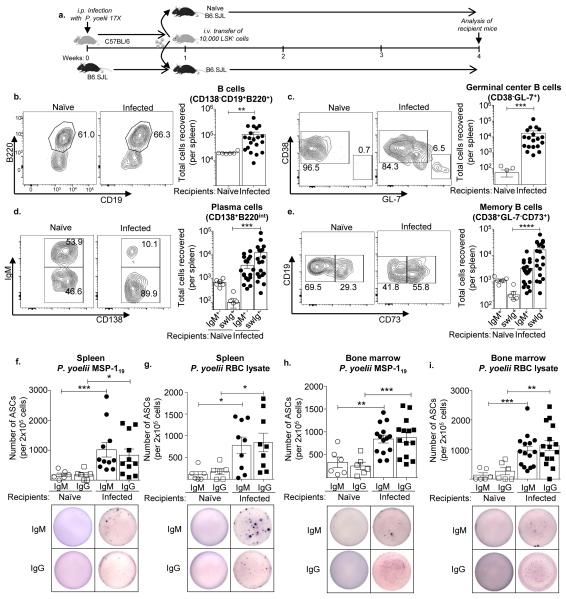
Figure 4. LSK− cell derived B cells produce parasite-specific antibodies.
a. LSK− cells were sorted from the spleen of CD45.2+ mice, one-week post P. yoelii 17X infection, and 10,000 cells were transferred into congenic B6.SJL (CD45.1) mice at same stage of infection (day 7). Three weeks later the spleens of recipient mice were harvested and stained with the indicated fluorochrome conjugated antibodies to sub-analyze the donor (CD45.2+) cell population. Representative flow plot analysis and total cell number of donor derived (CD45.2+) b. Total B cells (CD19+B220+), **, p=0.0033 c. Germinal center B cells (CD19+B220+GL7+CD38−), ***, p=0.0001 d. Plasma cells (CD138+B220lo/−) ***, p=0.0003 and e. Memory B cells (CD19+B220+CD38+GL-7−CD73+) ****, p<0.0001 (Mann-Whitney U test) in the spleen of recipient naïve (hollow circle) and Plasmodium yoelii 17X infected B6.SJL mice (filled circle), three weeks post-transfer. B cells were examined for CD138 and B220 expression and CD138+B220lo/− cells representing plasmablasts and plasma cells were gated on prior to analyzing IgM expression on these CD138+ cells. LSK− cells were sorted from the spleen of Ubc-GFP Tg mice, one-week post P. yoelii 17X infection, and 10,000 cells were transferred into naïve congenic B6.SJL (CD45.1) or B6.SJL mice at the same stage of infection (day 7). At week three post-transfer (day 28 p.i.), donor-derived GFP+ cells sorted from the spleen (f, g) and bone marrow (h, i) of recipient mice were used to determine the number of parasite-specific IgM (circle) or IgG (square) antibody secreting cells (ASCs) by ELISpot (P. yoelii MSP-119 (f, h) and P. yoelii infected RBC lysate (g, i)). *p<0.05, **p<0.001, ***p≤0.001 (Mann-Whitney U test). Representative ELISpot images from the assay are shown. Data are pooled from five (b-e) or four (f-i) independent experiments, n = 6-20 mice/group; Error bars (b-i) S.E.M.