Abstract
We investigated gene expression profiles of the NF-κB pathway in patients with triple-negative breast cancer (TNBC) receiving adjuvant chemotherapy to determine the prognostic value of NF-κB pathway genes according to chemotherapeutic regimen. We used the nCounter expression assay to measure expression of 11 genes (NFKB1, NFKB2, RELA, RELB, REL, TP53, FOXC1, TBP, SP1, STAT3 and IRF1 genes) belonging to the NF-κB pathway using mRNA extracted from paraffin-embedded tumor tissues from 203 patients diagnosed with TNBC. Of the 203 patients, 116 were treated with a chemotherapeutic regimen containing doxorubicin. As revealed by the expression profiles of the 11 genes, increased expression of SP1 was associated with poor prognosis in TNBC patients treated with adjuvant doxorubicin chemotherapy (5-year distant recurrence-free survival [5Y DRFS], low vs. high expression [cut-off: median]: 92.3% vs. 71.6%, P = 0.001). In a multivariate Cox regression model, SP1 expression was a useful marker for predicting long-term prognosis in TNBC patients receiving doxorubicin treatment, and we thus suggest that SP1 expression could serve as a prognostic marker in these patients.
Breast cancer is the most common cancer in women worldwide1. Of breast cancers, triple-negative breast cancer (TNBC), which is defined by the absence of hormone receptor expression and HER2 overexpression, has more aggressive biologic features and poor prognosis2,3,4. In addition, TNBC has heterogeneous histologic characteristics5 and is associated with complex genetic alterations6. However, in spite of therapeutic advances in the potential druggable genetic alterations of TNBC, such as MARK and PARP inhibitors and anti-PD-L1 antibody, no target molecules have been identified for TNBC. Accordingly, cytotoxic chemotherapy remains the most effective treatment strategy for TNBC.
Doxorubicin, a cytotoxic agent affiliated with anthracycline, is the most active agent used to treat breast cancer in neoadjuvant, adjuvant and palliative settings7,8,9. In the adjuvant setting, doxorubicin-containing chemotherapy significantly reduced breast cancer recurrence and mortality7,10,11. No targeted agents are available for TNBC in an adjuvant setting and thus conventional cytotoxic chemotherapy is particularly important.
Doxorubicin inhibits DNA and RNA synthesis by intercalation between DNA/RNA strand pairs12,13 and inhibits topoisomerase II enzyme, thus blocking DNA transcription and replication14. However, the DNA damage induced by doxorubicin activates the NF-κB pathway, leading to doxorubicin resistance in cancer cell lines, including in breast cancer cells15,16,17,18.
The transcription factor NF-κB family consists of five gene members: RELA (RelA, p65), RELB (RelB), REL (c-Rel), NFKB1 (NF-κB1, p105) and NFKB2 (NF-κB2, p100). NF-κB transcription factors bind as dimers to κB sites in enhancers and promoters of various genes to regulate transcription19,20, and are also tightly controlled at multiple regulatory element levels20,21. SP1 activates RELA22 and REL transcription23, and STAT3 is a transcription factor for RELA, RELB and NFKB224. Additional transcription factors include TBP and FOXC1 for NFKB1, TP53 for NFKB2 and IRF1 for REL transcription25. Moreover, a recent study showed that p53 depletion is required for NF-κB target gene activation by doxorubicin chemotherapy26.
These five family gene members control two main NF-κB pathways: the canonical pathway composed by RelA and the non-canonical pathway associated with RelB and NF-κB220. In breast cancer cells, the canonical pathway of NF-κB is associated with doxorubicin resistance26,27,28. Moreover, studies using human breast cancer tissue have shown that high nuclear RelA expression promotes activation of the canonical NF-κB pathway and is associated with poor prognosis26,29,30. However, these studies were conducted regardless of breast cancer molecular subtypes based on the presence or absence of hormone receptor expression or HER2 overexpression. Moreover, there has been no previous study on the NF-κB pathway and doxorubicin resistance in TNBC.
Previous reports suggested that methotrexate inhibits NF-κB activity31. Tumor cells with an inactivated NF-κB pathway are sensitive to 5-fluorouracil chemotherapy32, while a gastric cancer cell line with NF-κB pathway activation acquired 5-fluorouracil chemotherapy resistance33.
We conducted this study to reveal how transcription factor NF-κB family genes and their regulatory elements affect response to adjuvant chemotherapy, including doxorubicin, in patients with TNBC.
Results
Patient characteristics
A total of 203 TNBC patients who underwent curative surgery and adjuvant chemotherapy were enrolled (Supplementary Fig. 1 and Table 1). One patient was excluded due to missing chemotherapy regimen records. Of the remaining 202 patients, 116 were treated with doxorubicin-containing chemotherapy and 86 received cyclophosphamide, methotrexate and 5-fluorouracil (CMF) chemotherapy. Of the 116 patients, 58 (50.0%) were treated with doxorubicin, cyclophosphamide and 5-fluorouracil (FAC) chemotherapy, 17 (14.7%) received doxorubicin and cyclophosphamide (AC) and 41 (35.3%) received AC followed by taxane (AC-T) chemotherapy.
Table 1. Impact of baseline characteristics on patient prognosis (N = 203).
| Total N = 203 (%) | 5Y DRFS (%) | P-value | |
|---|---|---|---|
| Age (median) | 46.4 ± 10.2 | ||
| Range | 23.5–74.1 | 0.633 | |
| <40 YO1 | 48 (23.6) | 81.2 | |
| ≥40 YO | 155 (76.4) | 86.4 | |
| Histology | 0.507 | ||
| IDC2 | 180 (88.7) | 85.5 | |
| Other | 23 (11.3) | 82.9 | |
| Stage | <0.001 | ||
| I | 55 (27.1) | 90.9 | |
| IIA | 94 (46.3) | 91.5 | |
| IIB | 33 (16.3) | 78.8 | |
| IIIA | 13 (6.4) | 67.7 | |
| IIIB | 0 (0) | ||
| IIIC | 8 (3.9) | 25.0 | |
| Nuclear grade | 0.258 | ||
| 1 | 2 (1.0) | 50.0 | |
| 2 | 47 (23.2) | 82.8 | |
| 3 | 145 (71.4) | 86.9 | |
| Unknown | 9 (4.4) | 77.8 | |
| Histologic grade | 0.704 | ||
| 1 | 3 (1.5) | 100.0 | |
| 2 | 45 (22.2) | 84.4 | |
| 3 | 144 (70.9) | 86.0 | |
| Unknown | 11 (5.4) | 72.7 | |
| Adjuvant chemotherapy | 0.001 | ||
| CMF3 | 86 (42.4) | 90.7 | |
| FAC4 | 58 (28.6) | 86.0 | |
| AC5 | 17 (8.4) | 100.0 | |
| AC –T6 | 41 (20.2) | 65.9 | |
| Unknown | 1 (0.5) | 100.0 | |
| Adjuvant RTx5 | 0.093 | ||
| Yes | 130 (64.0) | 83.0 | |
| No | 73 (36.0) | 89.0 | |
| NFKB1 (median: 25.60) | 0.057 | ||
| Low | 101(49.9%) | 81.2 | |
| High | 102(50.1%) | 89.1 | |
| NFKB2 (median: 297.30) | 0.793 | ||
| Low | 101(49.9%) | 85.1 | |
| High | 102(50.1%) | 85.3 | |
| RELA (median: 224.85) | 0.061 | ||
| Low | 101(49.9%) | 90.0 | |
| High | 102(50.1%) | 80.4 | |
| RELB (median: 61.25) | 0.567 | ||
| Low | 101(49.9%) | 86.1 | |
| High | 102(50.1%) | 84.3 | |
| REL (median: 65.48) | 0.411 | ||
| Low | 101(49.9%) | 82.2 | |
| High | 102(50.1%) | 88.2 | |
| TP53 (median: 221.06) | 0.957 | ||
| Low | 101(49.9%) | 85.3 | |
| High | 102(50.1%) | 85.1 | |
| FOXC1 (median: 126.94) | 0.678 | ||
| Low | 101(49.9%) | 87.1 | |
| High | 102(50.1%) | 83.3 | |
| TBP (median: 92.68) | 0.307 | ||
| Low | 101(49.9%) | 87.1 | |
| High | 102(50.1%) | 83.3 | |
| SP1 (median: 100.01) | 0.024 | ||
| Low | 101(49.9%) | 90.1 | |
| High | 102(50.1%) | 80.3 | |
| STAT3 (median:1599.68) | 0.732 | ||
| Low | 101(49.9%) | 86.1 | |
| High | 102(50.1%) | 84.3 | |
| IRF1 (median: 192.75) | 0.758 | ||
| Low | 101(49.9%) | 86.1 | |
| High | 102(50.1%) | 84.3 |
1Years old, 2Invasive ductal carcinoma, 3Cyclophospamide/Methotrexate/Fluorouracil, 4Fluorouracil/Adriamycin/Cyclophosphamide, 5Adriamycin/Cyclophosphamide, 6Taxane, 7Radiotherapy.
The selection of chemotherapy regimen depended on diagnostic stage (P < 0.001) (Table 2). Most patients with stage I/IIA breast cancer were treated with CMF or FAC chemotherapy (61.8% and 29.1% in stage I and 48.9% and 34.0% in stage IIA, respectively), whereas patients with stage IIB/IIIA and IIIC breast cancer received AC-T chemotherapy (48.5% in stage IIB, 69.2% in stage IIIA and 87.5% in stage IIIC).
Table 2. Impact of baseline characteristics on patient prognosis according to adjuvant chemotherapy (N = 202).
| Doxorubicin N = 116 (%) | 5Y DRFS (%) | P-value | CMF1 N = 86 (%) | 5Y DRFS (%) | P-value | |
|---|---|---|---|---|---|---|
| Age (median) | 46.3 ± 9.7 | 0.534 | 46.4 ± 0.9 | 0.077 | ||
| Range | 28.3–74.1 | 23.5–73.1 | ||||
| <40 YO1 | 29 (25.0) | 82.6 | 19 (22.1) | 78.9 | ||
| ≥40 YO | 87 (75.0) | 80.4 | 67 (77.9) | 94.0 | ||
| Histology | 0.765 | 0.207 | ||||
| IDC2 | 108 (93.1) | 71 (82.6) | 91.5 | |||
| Other | 8 (6.9) | 15 (17.4) | 86.7 | |||
| Stage | <0.001 | 0.860 | ||||
| I | 21 (18.1) | 90.5 | 34 (39.5) | 91.2 | ||
| IIA | 48 (41.4) | 93.8 | 46 (53.5) | 89.1 | ||
| IIB | 26 (22.4) | 73.1 | 6 (7.0) | 100.0 | ||
| IIIA | 13 (11.2) | 67.7 | 0 (0) | NA | ||
| IIIB | 0 (0) | NA | 0 (0) | NA | ||
| IIIC | 8 (6.9) | 25.0 | 0 (0) | NA | ||
| Nuclear grade | 0.503 | 0.134 | ||||
| 1 | 0 (0) | NA | 2 (2.3) | 50.0 | ||
| 2 | 31 (26.7) | 80.3 | 16 (18.6) | 87.5 | ||
| 3 | 83 (71.6) | 81.9 | 61(71.0) | 93.4 | ||
| Unknown | 2 (1.7) | 50.0 | 7 (8.1) | 85.7 | ||
| Histologic grade | 0.898 | 0.395 | ||||
| 1 | 1 (0.9) | 100.0 | 2 (2.3) | 100.0 | ||
| 2 | 25 (21.5) | 84.0 | 20 (23.3) | 85.0 | ||
| 3 | 87 (75.0) | 79.1 | 56 (65.1) | 94.6 | ||
| Unknown | 3 (2.6) | 66.7 | 8 (9.3) | 75.0 | ||
| Adjuvant RTx3 | 0.052 | 0.814 | ||||
| Yes | 79 (68.1) | 77.1 | 51 (59.3) | 92.2 | ||
| No | 37 (31.9) | 89.2 | 35 (40.7) | 88.6 | ||
| NFKB1 (median: 25.60) | 0.034 | 0.948 | ||||
| Low | 62 (53.4) | 74.2 | 39 (45.3) | 92.3 | ||
| High | 54 (46.6) | 88.8 | 47 (54.7) | 89.4 | ||
| NFKB2 (median: 297.30) | 0.754 | 0.864 | ||||
| Low | 55 (47.4) | 81.7 | 46 (53.5) | 89.1 | ||
| High | 61 (52.6) | 80.3 | 40 (46.5) | 92.5 | ||
| RELA (median: 224.85) | 0.023 | 0.832 | ||||
| Low | 55 (47.4) | 90.8 | 45 (52.3) | 88.9 | ||
| High | 61 (52.6) | 72.1 | 41 (47.7) | 92.7 | ||
| RELB (median: 61.25) | 0.666 | 0.715 | ||||
| Low | 57 (49.1) | 82.3 | 44 (51.7) | 90.9 | ||
| High | 59 (50.9) | 79.6 | 42 (48.8) | 90.5 | ||
| REL (median: 65.48) | 0.460 | 0.716 | ||||
| Low | 67 (57.8) | 77.6 | 34 (39.5) | 91.2 | ||
| High | 49 (42.2) | 85.6 | 52 (60.5) | 90.4 | ||
| TP53 (median: 221.06) | 0.886 | 0.750 | ||||
| Low | 59 (50.9) | 79.7 | 42 (48.8) | 92.9 | ||
| High | 57 (49.1) | 82.3 | 44 (51.2) | 88.6 | ||
| FOXC1 (median: 126.94) | 0.208 | 0.279 | ||||
| Low | 59 (50.9) | 86.3 | 42 (48.8) | 88.1 | ||
| High | 57 (49.1) | 75.4 | 44 (51.2) | 93.2 | ||
| TBP (median: 92.68) | 0.609 | 0.158 | ||||
| Low | 61 (52.6) | 81.8 | 39 (45.3) | 94.9 | ||
| High | 55 (47.4) | 80.0 | 47 (54.7) | 87.2 | ||
| SP1 (median: 100.01) | 0.001 | 0.193 | ||||
| Low | 52 (44.8) | 92.3 | 49 (57.0) | 87.8 | ||
| High | 64 (55.2) | 71.6 | 37 (43.0) | 94.6 | ||
| STAT3 (median:1599.68) | 0.122 | 0.082 | ||||
| Low | 58 (50.0) | 86.1 | 43 (50.0) | 86.0 | ||
| High | 58 (50.0) | 75.9 | 43 (50.0) | 95.3 | ||
| IRF1 (median: 192.75) | 0.695 | 0.599 | ||||
| Low | 51 (44.0) | 82.2 | 50 (58.1) | 90.0 | ||
| High | 65 (56.0) | 80.0 | 36 (41.9) | 91.7 |
1Years old, 2Invasive ductal carcinoma, 3Radiotherapy.
The expression profile of NF-κB pathway-associated genes is described in Supplementary Table 1 and Supplementary Fig. 2. For further survival analysis, we set the median expression score of the 11 genes as the cut-off value to divide patients into two groups based on lower and higher expression.
NF-κB Pathway gene expression profile
Interactions among genes expressed in the NF-κB pathway are described in Supplementary Fig. 3 and we performed Pearson correlation analysis to explore associations among the expressed genes. The eleven NF-κB pathway-associated genes were classified into two categories: one consisted of NFKB1, NFKB2, REL, RELA and RELB (NF-κB pathway genes) and the other consisted of SP1, STAT3, TBP, FOXC1, IRF1 and TP53 (regulatory genes of NF-κB pathway genes).
Among the NF-κB pathway genes, NFKB1 expression was positively correlated with REL expression (Pearson R: 0.694, P < 0.001), and NFKB2 was positively correlated with RELA and RELB expression (Pearson R between NFKB2 and RELA: 0.825, P < 0.001 and Pearson R between NFKB2 and RELB: 0.728, P < 0.001; Supplementary Table 1).
Analysis of the association between the six regulatory genes and five NF-κB pathway genes revealed that higher SP1 or STAT3 expression was strongly correlated with RELA overexpression (Pearson R: 0.845, P < 0.001 of SP1 and 0.697, P < 0.001 of STAT3).
Impact of baseline characteristics, including NF-κB pathway gene expression, on distant recurrence-free survival according to chemotherapy regimen
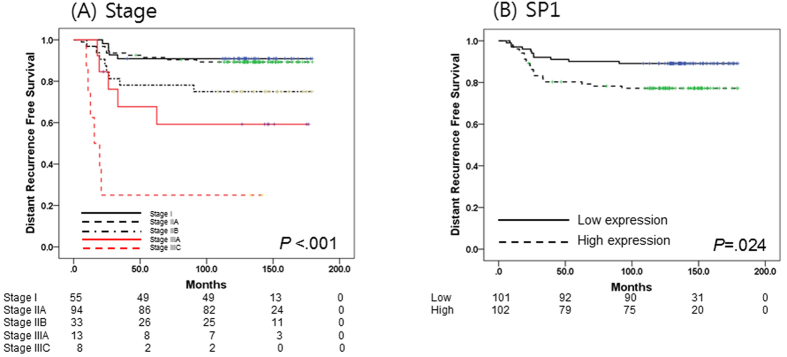
Of 203 patients, 34 patients (16.7%) experienced breast cancer recurrence. Median follow-up duration was 137 months. In univariate analysis, the five-year DRFS (5Y DRFS) rate in patients with stage I and IIA disease was 90.9% and 91.5%, respectively, compared to 78.8%, 67.7%, and 25.0% in patients with stage IIB, IIIA, and IIIC disease, respectively (P < 0.001) (Table 1 and Fig. 1A). Chemotherapy regimen affected DRFS (P = 0.001), but was also highly related to disease stage (P < 0.001) (Tables 1 and 2).
Figure 1. Survival analysis in patients with triple negative breast cancer (TNBC) (N = 203).
(A) Kaplan-Meier survival curve of stage at diagnosis. (B) Kaplan-Meier survival curve of SP1 expression.
Of NF-κB pathway-associated genes, the level of SP1 gene expression influenced DRFS in TNBC patients (5Y DRFS, low vs. high: 91.1% vs. 80.3%, P = 0.024) (Table 1 and Fig. 1B). In addition, the level of NFKB1 and RELA expression also marginally impacted DRFS (5Y DRFS, [low vs. high expression of NFKB1: 81.2% vs. 89.1%, P = 0.057], [low vs. high expression of RELA: 90.1% vs. 80.3%, P = 0.061]).
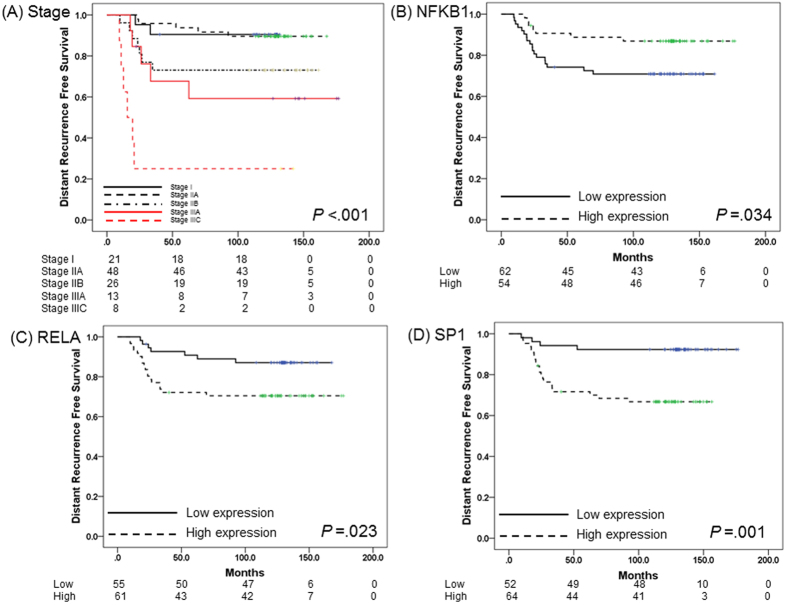
In subgroup analysis of patients who received doxorubicin chemotherapy, disease stage significantly affected DRFS. 5Y DRFS rates for stage I and IIA disease were 90.5% and 93.8%, respectively, in contrast with 73.1%, 67.7% and 25.0% for stage IIB, IIIA and IIIC, respectively (P < 0.001) (Table 2 and Fig. 2A). With regard to gene expression profile, low expression of NFKB1 and high expression of RELA and SP1 were associated with short DRFS duration ([5Y DRFS for NFKB1, low vs. high: 74.2% vs. 88.8%, P = 0.034], [5Y DRFS for RELA, low vs. high: 90.8% vs. 72.1%, P = 0.023], [5Y DRFS for SP1, low vs. high: 92.3% vs. 71.6%, P = 0.001]) (Table 1 and Fig. 2B–D).
Figure 2. Survival analysis in TNBC treated with adjuvant doxorubicin chemotherapy (N = 116).
(A) Kaplan-Meier survival curve of stage at diagnosis. (B) Kaplan-Meier survival curve of NFKB1 expression. (C) Kaplan-Meier survival curve of RELA expression. (D) Kaplan-Meier survival curve of SP1 expression.
Furthermore, we performed multivariate analysis of factors associated with patient prognosis in univariate analysis, including stage, chemotherapeutic agent and NFKB1, RELA and SP1 gene expression in patients with doxorubicin chemotherapy. Stage and expression level of SP1 remained statistically significant factors affecting DRFS ([hazard ratio (HR) of DRFS 1.34 (95% confidence interval [CI] 0.26–6.95) for stage IIA, 3.60 (95% CI 0.75–17.36) for stage IIB, 7.07 (95% CI 1.36–36.91) for stage IIIA, and 20.19 (95% CI 4.00–101.87) for IIIC, P < 0.001], [HR of DRFS 4.97 (95% CI 1.68–14.73) for high SP1 expression, P = 0.004]) (Table 3).
Table 3. Impact of NFKB family mRNA expression levels on DRFS in patients with TNBC who received adjuvant doxorubicin chemotherapy (Cox regression).
| Doxorubicin chemotherapy | |||
|---|---|---|---|
| Clinical variables (N = 116) | HR | 95% CI | P-value |
| Stage | <0.001 | ||
| I | 1.0 | NA | |
| IIA | 1.34 | 0.26–6.95 | |
| IIB | 3.60 | 0.75–17.36 | |
| IIIA | 7.07 | 1.36–36.91 | |
| IIIC | 20.19 | 4.00–101.87 | |
| Adjuvant chemotherapy | 0.664 | ||
| (F)AC | 1.0 | NA | |
| AC-T | 0.79 | 0.27–2.33 | |
| NFKB1 (Median: 25.60) | 0.527 | ||
| Low | 1.0 | NA | |
| High | 0.73 | 0.27–1.96 | |
| RELA (Median: 224.85) | 0.823 | ||
| Low | 1.0 | NA | |
| High | 0.88 | 0.27–2.83 | |
| SP1 (Median: 100.01) | 0.004 | ||
| Low | 1.0 | NA | |
| High | 4.97 | 1.68–14.73 | |
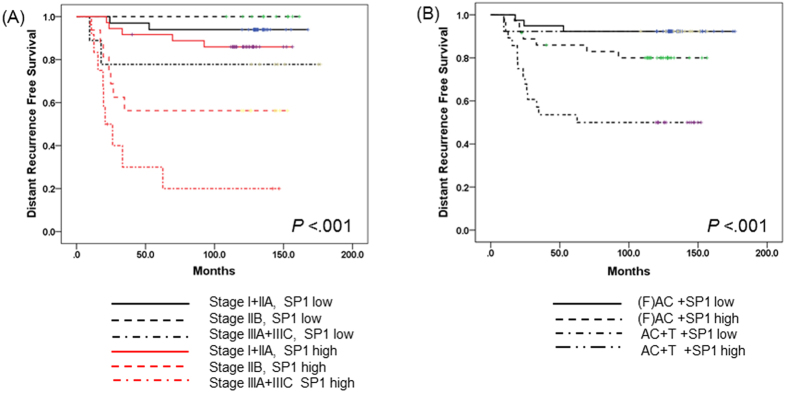
The effect of SP1 expression on DRFS was more intensive in advanced-stage TNBC patients treated with adjuvant doxorubicin chemotherapy. For stage IIIA and IIIC, the 5Y DRFS rate in patients with high SP1 expression was 30.0%, compared to 77.8% in patients with low SP1 expression. Patients who overexpressed SP1 with stage I/IIA and IIB TNBC had 5Y DRFS of 91.7% and 56.3%, respectively, while those with low expression of SP1 had 5Y DRFS of 93.9% and 100.0%, respectively (P < 0.001) (Fig. 3A). In addition, we analyzed the co-effect of taxane chemotherapy and SP1 expression on survival. In this analysis, regardless of additional docetaxel treatment, high SP1 expression was significantly associated with DRFS ([5Y DRFS in high SP1 expression, (F)AC and AC+T chemotherapy: 85.9% and 53.6%], [low SP1 expression: 92.3% and 92.3%], P < 0.001) (Fig. 3B). We examined the impact of baseline characteristics on DRFS in patients who received adjuvant CMF chemotherapy, but SP1 expression had no significant effect on DRFS (Table 2).
Figure 3.
(A) Survival analysis according to stage and SP1 expression. (B) Survival analysis according to chemotherapy and SP1 expression.
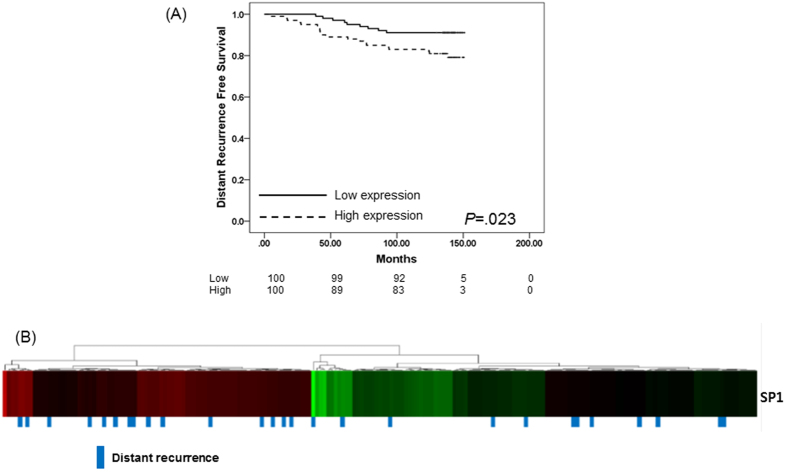
Lastly, we validated the impact of SP1 expression on breast cancer disease progression. Using breast cancer tissue from 202 patients diagnosed with breast cancer since 2003 to 2004, we tested the level of SP1 mRNA expression and analyzed the effect of SP1 expression level on DRFS. Analysis indicated that high SP1 mRNA expression was associated with distant recurrence of breast cancer (P = 0.023) (Fig. 4A,B). Median follow-up duration was 139.8 months and 29 patients (14.4%) had distant recurrence of cancer; 20 patients (19.8%) with high SP1 expression had tumor recurrence in contrast to only nine patients (8.9%) with low SP1 expression.
Figure 4. Survival analysis in the validation cohort (N = 202).
(A) Kaplan-Meier curve of distant recurrence-free survival according to SP1 expression. (B) Heat map for SP1 mRNA expression and distant recurrence status.
Predictive value of SP1 expression in TNBC patients treated with doxorubicin
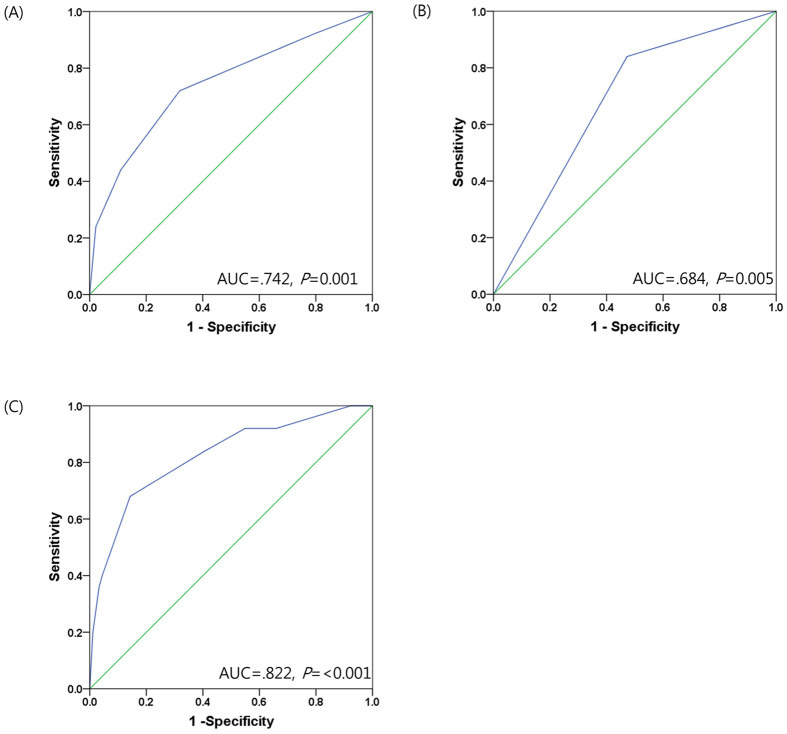
Receiver operating characteristics(ROC) analysis was performed to evaluate the predictive value of SP1 expression level. Adding SP1 expression level to TNM stage enabled prediction of DRFS in patients who received adjuvant doxorubicin chemotherapy. The results of ROC analysis revealed that SP1 expression strengthened the predictive efficacy of TNM stage ([TNM stage: AUC: 0.742, P = 0.001], [SP1 expression: AUC: 0.684, P = 0.005] and [TNM stage + SP1 expression: AUC: 0.822, P < 0.001]) (Fig. 5).
Figure 5. ROC analysis of the predictive accuracy of stage and SP1 expression for distant recurrence-free survival of patients with TNBC receiving adjuvant doxorubicin chemotherapy (N = 116).
(A) ROC curve of stage. (B) ROC curve of SP1 expression. (C) ROC curve of stage and SP1 expression.
Discussion
In this study, we demonstrated the impact of NF-κB pathway gene expression on the prognosis of TNBC and found that SP1 gene expression level was a potential prognostic marker in TNBC patients receiving adjuvant doxorubicin chemotherapy.
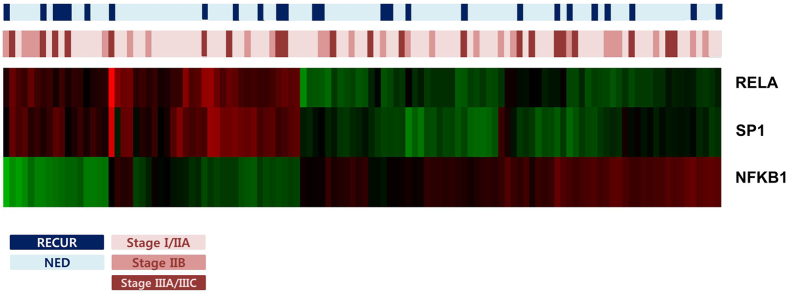
Many previous studies have shown that RelA expression is a predictive marker for doxorubicin chemotherapy26,29,30 by examining intra-nuclear staining using RelA antibody and detecting protein expression using intra-nuclear RelA expression score. In our study, we performed the nCounter expression assay to measure the expression of eleven genes: five canonical and non-canonical NF-κB pathway genes and six associated regulatory factors. With this approach, we sensitively detected gene expression regardless of intra-nuclear or extra-nuclear expression. In our results, univariate analysis revealed that three canonical NF-κB pathway genes, SP1, RELA and NFKB134, were related to doxorubicin resistance (Fig. 6).
Figure 6. Heatmap for SP1, RELA and NFKB1 expression.
Of these three genes, only SP1 maintained statistical significance in multivariate analysis, although SP1 and RELA expression were strongly associated; patients with high expression of SP1 also exhibited high RELA expression.
Sp1, part of the Sp/KLF family of transcription factors, is a zinc finger transcription factor that binds to GC-rich motifs of many promoters35. Several genes, including RelA, are regulated by Sp1 and high Sp1 expression promotes cell growth, cell survival and gene expression, causing carcinogenesis. Sp1 overexpression is a negative prognostic marker in pancreatic cancer and gastric cancer36,37 and Sp1 downregulation inhibits cell survival of rhabdomyosarcoma38. In addition, Sp1 overexpression induces doxorubicin resistance in HL-60, a myeloid leukemia cell line39.
Sp1 is a downstream target of many pathways in addition to NF-kB, including MAPK, and JNK pathways. SP1 and the other 10 measured genes are also involved in many genetic pathways. However, we only evaluated Sp1 and NF-kB pathway genes and did not perform pathway activity analysis. Therefore, our suggestion that SP1 and NFkB activation induced anthracycline resistance had limitation.
However, many previous studies already showed that canonical NF-kB pathway activation was positively correlated to anthracycline resistance using pathway analysis. Additionally, RELA and SP1 expression patterns interacted with each other and high SP1 and RELA expression indicated a high recurrence rate of TNBC. This result was similar to that of previous studies on the relationship between anthracycline resistance and canonic NF-kB pathway activation. Accordingly, we might suggest that the level of SP1 expression indicated canonic NF-kB pathway activity and thus is a potential prognostic marker of TNBC treated with anthracycline.
Multiple myeloma is a well-known hematologic malignancy that is regulated by Sp1 transactivation and the NF-κB pathway, and downregulation of Sp1 and RelA induces tumor regression40. Moreover, bortezomib, a gangbuster drug for multiple myeloma41, is a potent, highly selective, and reversible proteasome inhibitor that targets the 26S proteasome complex and inhibits its function. Besides inhibiting NF-κB, bortezomib inhibited tumor cell growth by targeting cell cycle regulatory proteins, the unfolded protein response (UPR) pathway, p53-mediated apoptosis, and DNA repair mechanisms as well as classical stress response pathways. Bortezomib suppresses Sp1 activity and disrupts the physical interaction of Sp1/RelA, ultimately downregulating the NF-kB pathway42. Accordingly, bortezomib represents a potential therapeutic strategy for anthracycline-resistant TNBC by activating Sp1 and NF- kB genes. Although a previous phase I/II study of bortezomib and capecitabine in patients with metastatic breast cancer found a moderate antitumor effect in heavily pretreated patients43, breast cancer patients with an activated NF-κB pathway might benefit from bortezomib treatment.
TP53 is a tumor suppressor gene; some reports have indicated that silencing of p53 expression is associated with anthracycline resistance in breast cancer26,44. However, we did not find any relationship between TP53 expression and prognosis. In addition, TP53 expression did not affect the expression of NF-κB pathway genes.
Our study is the first to demonstrate the impact of NF-κB pathway gene expression in TNBC patients treated with adjuvant doxorubicin chemotherapy. Our findings suggest that Sp1 expression is positively correlated with RelA expression. Moreover, high expression of Sp1 is associated with poor prognosis in patients with TNBC treated with adjuvant anthracycline chemotherapy. Considering the heterogeneous molecular characteristics of TNBC, Sp1 is not only a potential predictor of anthracycline response, but also a classification factor for clarifying the heterogeneity of TNBC. Furthermore, accurate prediction of anthracycline chemotherapy outcomes could change current practice guideline of adjuvant chemotherapy in TNBC45. Moreover, bortezomib, a proteasome inhibitor, is a potential therapeutic strategy for anthracycline-resistant TNBC through its activation of Sp1 and NF- kB genes.
Methods
Patients
This study was conducted via retrospective analysis of the clinical records of patients with invasive breast cancer who received adjuvant chemotherapy after curative surgery at Samsung Medical Center between 2000 and 2004. Women diagnosed with stage I to IIIC breast cancer were included. At initial diagnosis, medical history, physical examination, blood tests, mammography, breast ultrasonography, breast magnetic resonance imaging (MRI), abdominal computed tomography (CT) scan, and bone scans and/or positron emission tomography (PET)-CT scans (if indicated) were performed (Fig. 1).
The institutional review board of Samsung Medical Center, Seoul, Korea approved our study protocol and waived the need for informed consent due to the use of archival tissue samples and retrospective clinical data (IRB No: 2012-08-065).
RNA extraction
We examined hematoxylin and eosin (H&E)-stained slides from all available archival formalin-fixed, paraffin-embedded (FFPE) primary breast tumor tissue. Two independent pathologists reviewed all pathology specimens to determine the following tumor characteristics: histological grade46, nuclear grade, ER/progesterone receptor (PgR) expression and HER2 overexpression.
RNA was extracted from two to four 4-μm-thick FFPE sections that contained tumor tissue using the High Pure RNA Paraffin kit (Roche Diagnostic, Mannheim, Germany). RNA yield and purity were assessed using a NanoDrop ND-1000 Spectrophotometer (NanoDrop Technologies, Rockland, DE, USA). Samples with a total RNA concentration less than 50 ng/uL were excluded from analysis, because 200 ng of input RNA in 5 ul was used for hybridization with 20 uL of probe set master mix.
nCounter expression assay (NanoString)
The NanoString nCounter Analysis System (NanoString Technologies, Seattle, WA, USA) was used to measure the amount of gene expression. Using a multiplexed hybridization assay and digital readouts of fluorescent probes47, this system measures the relative abundance of each mRNA transcript. We used an nCounter CodeSet (NanoString Technologies) containing biotinylated capture probes for NFKB1, NFKB2, RELA, RELB, REL, TP53, FOXC1, TBP, SP1, STAT3 and IRF1 genes and 5 housekeeping genes and reporter probes attached to color barcode tags according to the nCounter code-set design. The CodeSet was hybridized in solution to 200 ng of total RNA for 18 h at 65 °C according to the manufacturer’s instructions.
Hybridized samples were loaded into the nCounter Prep Station for post hybridization processing. On the deck of the Prep Station, hybridized samples were purified and immobilized in a sample cartridge for data collection, followed by quantification of target mRNA in each sample using the nCounter Digital Analyzer. Quantified expression data were analyzed using NanoString’s nSolver Analysis Software.
After performing image quality control using a predefined cutoff value, we excluded outlier samples using a normalization factor based on the sum of positive control counts greater than threefold. Counts of the probes were then normalized using the geometric mean of the five housekeeping genes and log2 transformed for further analysis.
Statistical analysis
DRFS was defined as the elapsed time from the date of curative surgery to the detection of disease recurrence. DRFS was analyzed using the Kaplan-Meier method. Univariate and multivariate analyses of DRFS were performed using Cox’s proportional hazards regression tests. To evaluate the relationship among expression of the eleven genes, we use Pearson correlation analysis. Lastly, ROC analysis was performed to evaluate the prognostic value of the expression level of NF-kB family genes and ligands adding weight. ROC analysis was conducted by adding the weighted value of gene expression on a pre-existing known prognostic marker already validated using univariate analysis. Two-tailed P values of <0.05 were considered statistically significant and IBM SPSS Statistics 21 for Windows (IBM Corp., Armonk, NY, USA) was used to analyze all data.
Remark guidelines
We have adhered to the guidelines of a methodological paper from 2005 entitled “Reporting recommendations for tumor marker prognostic studies (REMARK guidelines)”48,49. To minimize any potential bias arising from review of medical records, we included “Patient Cohort” analysis to fulfill these criteria (Supplementary Figure 1).
Additional Information
How to cite this article: Kim, J.-Y. et al. The relationship between nuclear factor (NF)-κB family gene expression and prognosis in triple-negative breast cancer (TNBC) patients receiving adjuvant doxorubicin treatment. Sci. Rep. 6, 31804; doi: 10.1038/srep31804 (2016).
Supplementary Material
Acknowledgments
This research was funded by the Samsung Biomedical Research Institute (SMO1131841).
Footnotes
Author Contributions Y.H.P. conceived the study design, oversaw its conduct. J.-Y.K. wrote and revised this manuscript. H.H.J. conducted experiments and analyzed the data. H.H.J. and J.-Y.K. statistically analyzed and interpreted the data. S.A. contributed to pathologic review. S.Y.B., S.K.L., S.W.K., J.E.L. and S.J.N. collected samples. J.S.A. and Y.-H.I. collected clinical data.
References
- Howlader N. et al. SEER Cancer Statistics Review, 1975–2012. (National Cancer Institute, 2015). [Google Scholar]
- Reis-Filho J. S. & Tutt A. N. Triple negative tumours: a critical review. Histopathology 52, 108–118 (2008). [DOI] [PubMed] [Google Scholar]
- Foulkes W. D., Smith I. E. & Reis-Filho J. S. Triple-negative breast cancer. N Engl J Med 363, 1938–1948 (2010). [DOI] [PubMed] [Google Scholar]
- Dent R. et al. Triple-negative breast cancer: clinical features and patterns of recurrence. Clin Cancer Res 13, 4429–4434 (2007). [DOI] [PubMed] [Google Scholar]
- Metzger-Filho O. et al. Dissecting the heterogeneity of triple-negative breast cancer. J Clin Oncol 30, 1879–1887 (2012). [DOI] [PubMed] [Google Scholar]
- Nik-Zainal S. et al. Landscape of somatic mutations in 560 breast cancer whole-genome sequences. Nature (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Minckwitz G. et al. Doxorubicin with cyclophosphamide followed by docetaxel every 21 days compared with doxorubicin and docetaxel every 14 days as preoperative treatment in operable breast cancer: the GEPARDUO study of the German Breast Group. J Clin Oncol 23, 2676–2685 (2005). [DOI] [PubMed] [Google Scholar]
- Fisher B. et al. Two months of doxorubicin-cyclophosphamide with and without interval reinduction therapy compared with 6 months of cyclophosphamide, methotrexate, and fluorouracil in positive-node breast cancer patients with tamoxifen-nonresponsive tumors: results from the National Surgical Adjuvant Breast and Bowel Project B-15. J Clin Oncol 8, 1483–1496 (1990). [DOI] [PubMed] [Google Scholar]
- Gasparini G., Dal Fior S., Panizzoni G. A., Favretto S. & Pozza F. Weekly epirubicin versus doxorubicin as second line therapy in advanced breast cancer. A randomized clinical trial. Am J Clin Oncol 14, 38–44 (1991). [DOI] [PubMed] [Google Scholar]
- Early Breast Cancer Trialists’ Collaborative Group. Polychemotherapy for early breast cancer: an overview of the randomised trials. Lancet (London, England) 352, 930–942 (1998). [PubMed] [Google Scholar]
- Mamounas E. P. et al. Paclitaxel after doxorubicin plus cyclophosphamide as adjuvant chemotherapy for node-positive breast cancer: results from NSABP B-28. J Clin Oncol 23, 3686–3696 (2005). [DOI] [PubMed] [Google Scholar]
- Momparler R. L., Karon M., Siegel S. E. & Avila F. Effect of adriamycin on DNA, RNA, and protein synthesis in cell-free systems and intact cells. Cancer Res 36, 2891–2895 (1976). [PubMed] [Google Scholar]
- Fornari F. A., Randolph J. K., Yalowich J. C., Ritke M. K. & Gewirtz D. A. Interference by doxorubicin with DNA unwinding in MCF-7 breast tumor cells. Mol Pharmacol 45, 649–656 (1994). [PubMed] [Google Scholar]
- Tewey K. M., Rowe T. C., Yang L., Halligan B. D. & Liu L. F. Adriamycin-induced DNA damage mediated by mammalian DNA topoisomerase II. Science 226, 466–468 (1984). [DOI] [PubMed] [Google Scholar]
- Fang X. J. et al. Doxorubicin induces drug resistance and expression of the novel CD44st via NF-kappaB in human breast cancer MCF-7 cells. Oncol Rep 31, 2735–2742 (2014). [DOI] [PubMed] [Google Scholar]
- Jeremias I. et al. Inhibition of nuclear factor kappaB activation attenuates apoptosis resistance in lymphoid cells. Blood 91, 4624–4631 (1998). [PubMed] [Google Scholar]
- McDade T. P., Perugini R. A., Vittimberga F. J. Jr., Carrigan R. C. & Callery M. P. Salicylates inhibit NF-kappaB activation and enhance TNF-alpha-induced apoptosis in human pancreatic cancer cells. J Surg Res 83, 56–61 (1999). [DOI] [PubMed] [Google Scholar]
- Yi S. Y. et al. Favorable response to doxorubicin combination chemotherapy does not yield good clinical outcome in patients with metastatic breast cancer with triple-negative phenotype. BMC Cancer 10, 527 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pahl H. L. Activators and target genes of Rel/NF-kappaB transcription factors. Oncogene 18, 6853–6866 (1999). [DOI] [PubMed] [Google Scholar]
- Hayden M. S. & Ghosh S. Signaling to NF-kappaB. Genes Dev 18, 2195–2224 (2004). [DOI] [PubMed] [Google Scholar]
- Oeckinghaus A., Hayden M. S. & Ghosh S. Crosstalk in NF-kappaB signaling pathways. Nat Immunol 12, 695–708 (2011). [DOI] [PubMed] [Google Scholar]
- Hirano F. et al. Functional interference of Sp1 and NF-kappaB through the same DNA binding site. Mol Cell Biol 18, 1266–1274 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sif S. & Gilmore T. D. Interaction of the v-Rel oncoprotein with cellular transcription factor Sp1. Journal of virology 68, 7131–7138 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grivennikov S. I. & Karin M. Dangerous liaisons: STAT3 and NF-kappaB collaboration and crosstalk in cancer. Cytokine Growth Factor Rev 21, 11–19 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaslavsky E. et al. Antiviral response dictated by choreographed cascade of transcription factors. J Immunol 184, 2908–2917 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalmases A. et al. Deficiency in p53 is required for doxorubicin induced transcriptional activation of NF-small ka, CyrillicB target genes in human breast cancer. Oncotarget 5, 196–210 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapia M. A. et al. Inhibition of the canonical IKK/NF kappa B pathway sensitizes human cancer cells to doxorubicin. Cell Cycle 6, 2284–2292 (2007). [DOI] [PubMed] [Google Scholar]
- Li Y. et al. Inactivation of nuclear factor kappaB by soy isoflavone genistein contributes to increased apoptosis induced by chemotherapeutic agents in human cancer cells. Cancer Res 65, 6934–6942 (2005). [DOI] [PubMed] [Google Scholar]
- Montagut C. et al. Activation of nuclear factor-kappa B is linked to resistance to neoadjuvant chemotherapy in breast cancer patients. Endocr Relat Cancer 13, 607–616 (2006). [DOI] [PubMed] [Google Scholar]
- Buchholz T. A. et al. The nuclear transcription factor kappaB/bcl-2 pathway correlates with pathologic complete response to doxorubicin-based neoadjuvant chemotherapy in human breast cancer. Clin Cancer Res 11, 8398–8402 (2005). [DOI] [PubMed] [Google Scholar]
- Majumdar S. & Aggarwal B. B. Methotrexate suppresses NF-kappaB activation through inhibition of IkappaBalpha phosphorylation and degradation. J Immunol 167, 2911–2920 (2001). [DOI] [PubMed] [Google Scholar]
- Voboril R. et al. Inhibition of NF-kappa B augments sensitivity to 5-fluorouracil/folinic acid in colon cancer. J Surg Res 120, 178–188 (2004). [DOI] [PubMed] [Google Scholar]
- Camp E. R. et al. Inducible nuclear factor-kappaB activation contributes to chemotherapy resistance in gastric cancer. J Am Coll Surg 199, 249–258 (2004). [DOI] [PubMed] [Google Scholar]
- Park Y. H. et al. A seven-gene signature can predict distant recurrence in patients with triple-negative breast cancers who receive adjuvant chemotherapy following surgery. Int J Cancer 136, 1976–1984 (2015). [DOI] [PubMed] [Google Scholar]
- Safe S. & Abdelrahim M. Sp transcription factor family and its role in cancer. Eur J Cancer 41, 2438–2448 (2005). [DOI] [PubMed] [Google Scholar]
- Jiang N. Y. et al. Sp1, a new biomarker that identifies a subset of aggressive pancreatic ductal adenocarcinoma. Cancer Epidemiol Biomarkers Prev 17, 1648–1652 (2008). [DOI] [PubMed] [Google Scholar]
- Wang L. et al. Transcription factor Sp1 expression is a significant predictor of survival in human gastric cancer. Clin Cancer Res 9, 6371–6380 (2003). [PubMed] [Google Scholar]
- Chadalapaka G. et al. Inhibition of rhabdomyosarcoma cell and tumor growth by targeting specificity protein (Sp) transcription factors. Int J Cancer 132, 795–806 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borellini F., Aquino A., Josephs S. F. & Glazer R. I. Increased expression and DNA-binding activity of transcription factor Sp1 in doxorubicin-resistant HL-60 leukemia cells. Mol Cell Biol 10, 5541–5547 (1990). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulciniti M. et al. Significant biological role of sp1 transactivation in multiple myeloma. Clin Cancer Res 17, 6500–6509 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson P. G. et al. Bortezomib or high-dose dexamethasone for relapsed multiple myeloma. N Engl J Med 352, 2487–2498 (2005). [DOI] [PubMed] [Google Scholar]
- Liu S. et al. Bortezomib induces DNA hypomethylation and silenced gene transcription by interfering with Sp1/NF-kappaB-dependent DNA methyltransferase activity in acute myeloid leukemia. Blood 111, 2364–2373 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid P. et al. A phase I/II study of bortezomib and capecitabine in patients with metastatic breast cancer previously treated with taxanes and/or anthracyclines. Ann Oncol 19, 871–876 (2008). [DOI] [PubMed] [Google Scholar]
- Rahko E., Blanco G., Soini Y., Bloigu R. & Jukkola A. A mutant TP53 gene status is associated with a poor prognosis and anthracycline-resistance in breast cancer patients. Eur J Cancer 39, 447–453 (2003). [DOI] [PubMed] [Google Scholar]
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®): Breast Cancer Version3. http://www.nccn.org/professionals/physician_gls/f_guidelines.asp#site. Accessed 1 Sep 2015. (2015).
- Bloom H. J. & Richardson W. W. Histological grading and prognosis in breast cancer; a study of 1409 cases of which 359 have been followed for 15 years. Br J Cancer 11, 359–377 (1957). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiss G. K. et al. Direct multiplexed measurement of gene expression with color-coded probe pairs. Nat Biotechnol 26, 317–325 (2008). [DOI] [PubMed] [Google Scholar]
- McShane L. M. et al. Reporting recommendations for tumor marker prognostic studies. J Clin Oncol 23, 9067–9072 (2005). [DOI] [PubMed] [Google Scholar]
- McShane L. M. et al. REporting recommendations for tumor MARKer prognostic studies (REMARK). Breast Cancer Res Treat 100, 229–235 (2006). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.