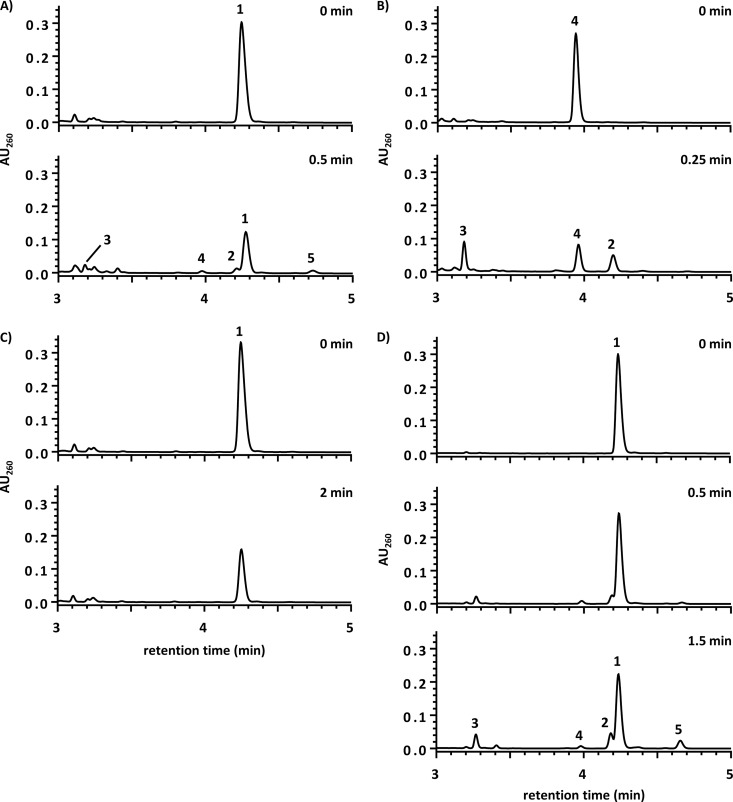
FIG 3 .
Selected HPLC diagrams demonstrating the conversion of aromatic CoA esters by cell extracts of T. aromatica grown with 4-F-benzoate (A to C) and by purified 4-F-BCR (D). All assays contained Ti(III) citrate (5 mM) and 0.2 mM of the individual CoA ester substrates. Conversion of CoA esters by cell extracts: 4-F-BzCoA in the presence of MgATP (A), BzCoA in the presence of MgATP (B), 4-F-BzCoA without MgATP (C), 4-F-BzCoA by purified 4-F-BCR (contaminated by cyclohexa-1,5-diene-1-carboxyl-CoA hydratase) (D). The loss of peak areas was due to thioesterase activity of the extracts that was subtracted from the activities determined. Compounds are indicated in the figure by numbers above the peaks as follows: 1, 4-F-BzCoA; 2, 1,5-dienoyl-CoA; 3, 6-OH-monoenoyl-CoA; 4, BzCoA; 5, 1-monoenoyl-CoA. An additional minor peak eluting at 3.4 min in panels A and D is assigned to 2-hydroxycyclohexanecarboxyl-CoA, which is formed from compound 5 by a side reaction of 1,5-dienoyl-CoA hydratase present in cell extracts and as a highly active minor contamination in purified BCR. AU260, relative absorption units at 260 nm.