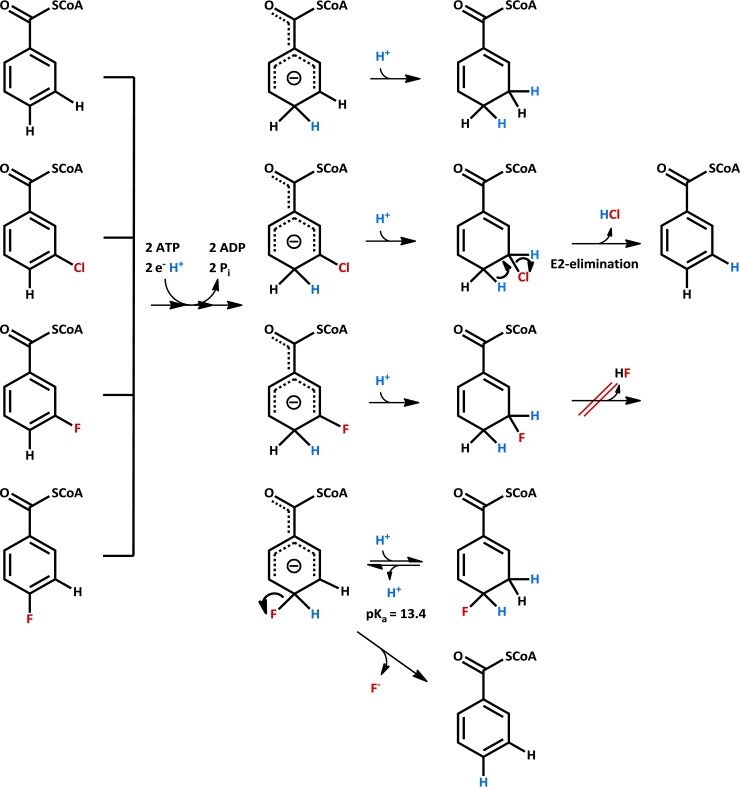
FIG 6 .
Possible mechanisms for the conversion of BzCoA and halogenated analogues by ATP-dependent class I BCR. A Birch-like mechanism involving two single ATP-dependent electron transfer steps and one protonation step yielding an anionic state is suggested for all shown BzCoA analogues. In the cases of BzCoA, 3-Cl-BzCoA, and 3-F-BzCoA, protonation of the anionic state is essentially irreversible, forming the corresponding dienoyl-CoA compounds. In the case of 3-Cl-BzCoA, but not 3-F-BzCoA, the halogenated dienoyl-CoA spontaneously eliminates HCl in an E2 manner. During conversion of 4-F-BzCoA, the pKa of the assumed 4-F-dienoyl-CoA intermediate (C-3 position) is significantly decreased. As a result, the essentially irreversible fluoride release in an E1cB-type elimination is driven by rearomatization and favored over 4-F-dienoyl-CoA formation.