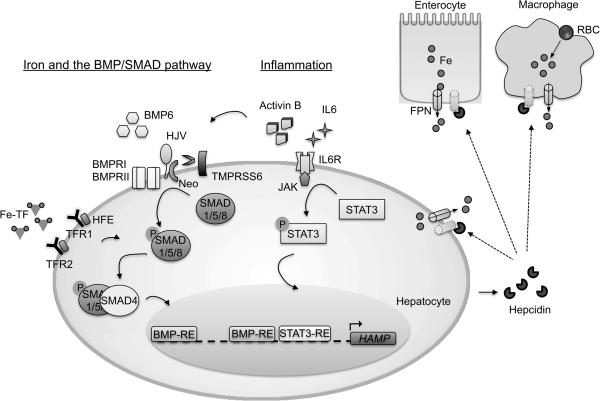
Figure 1. Current model of hepcidin regulation by iron and inflammation.
Iron stimulates hepcidin (HAMP) transcription through holo-transferrin (Fe-TF) and BMP6. Liver iron increases BMP6 expression in nonparenchymal cells through an unknown mechanism. Fe-TF is sensed by binding to transferrin receptor 1 (TFR1) and transferrin receptor 2 (TFR2). The hemochromatosis protein HFE is displaced from TFR1 by Fe-Tf binding. HFE and TFR2 functionally intersect with the BMP-SMAD1/5/8 pathway to modulate hepcidin transcription through mechanisms that are still being worked out, but may involve interactions with the BMP co-receptor hemojuvelin (HJV) and/or the BMP type I receptor ALK3. BMP6 binding to HJV, type II receptors (BMPRII) and type I receptors (BMPRI) induces phosphorylation of SMAD1/5/8 proteins, which complex with SMAD4 and translocate to the nucleus to bind 2 BMP responsive elements (BMP-RE) on the HAMP promoter, thereby inducing transcription. TMPRSS6 cleaves HJV to reduce BMP-SMAD1/5/8 signaling in response to iron deficiency. Neogenin (Neo) is an HJV interacting protein that may also be involved in hepcidin regulation. Inflammatory stimuli induce expression of IL6 and Activin B, which activate the JAK/STAT3 and BMPR/SMAD pathways respectively to induce hepcidin transcription. Hepcidin promotes the degradation of ferroportin (FPN) in enterocytes, macrophages, and hepatocytes to limit iron entry into the bloodstream.