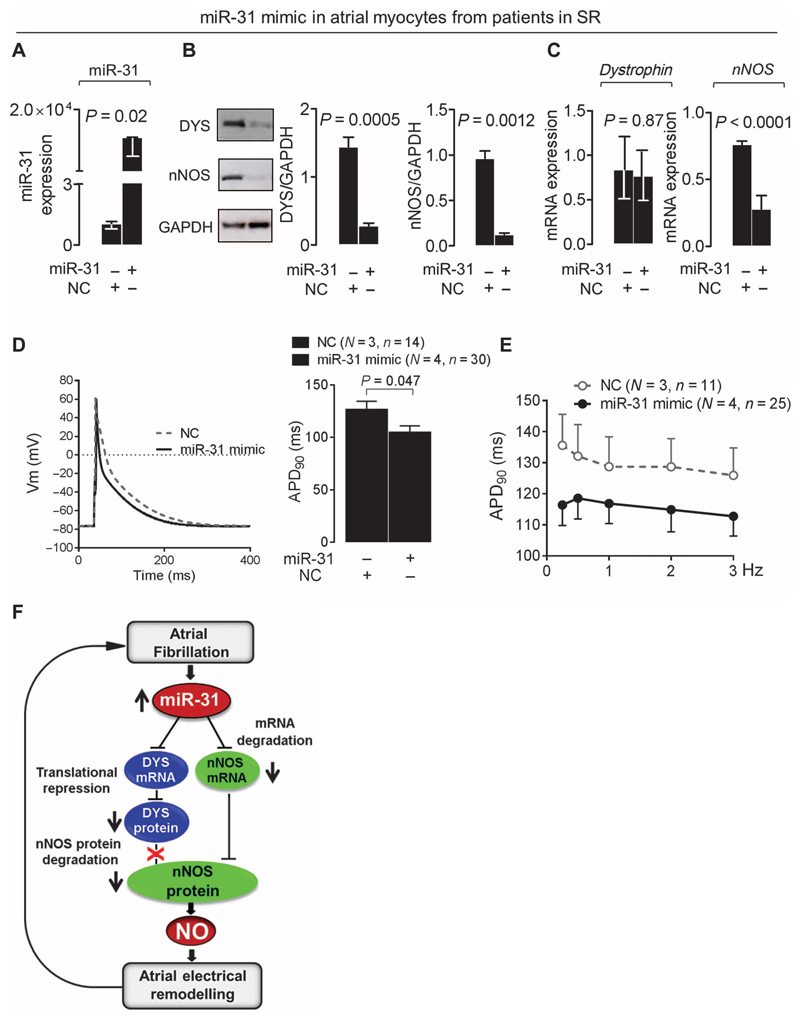
Fig. 7. miR-31 overexpression depletes nNOS and dystrophin and shortens APD in SR.
(A to C) Effect of miR-31 overexpression with an miR-31 mimic [versus nontargeting control (NC) (A)] on dystrophin and nNOS protein [n = 5 per group (B)] or mRNA [n = 5 to 9 (C)] in atrial myocytes from patients in SR. Data are averages ± SEM except for miR-31 (A) and dystrophin (C), where they are medians and interquartile ranges. P values were determined by unpaired t test or Mann-Whitney U test [miR-31 in (A) and dystrophin in (C)]. (D) APD90 in SR atrial myocytes treated with an miR-31 mimic or NC. Left: Representative data traces. Right: Average values ± SEM. P value was determined by unpaired t test. (E) APD90 rate-dependent adaptation in SR myocytes treated with an miR-31 mimic or NC. Data are averages ± SEM. P = 0.227 for the effect of treatment with the miR-31 mimic, and P = 0.378 for the interaction between treatment and frequency by two-way repeated-measures ANOVA. In (D) and (E), N is the number of patients, and n is the number of cells. (F) Summary diagram of the main findings: atrial miR-31 up-regulation in atrial myocytes from patients with AF accelerates nNOS mRNA decay and alters nNOS subcellular localization and protein stability by inhibiting dystrophin translation. The resulting reduction in nNOS-derived NO contributes to the AF-induced electrical remodeling and, thus, to the maintenance and progression of AF.