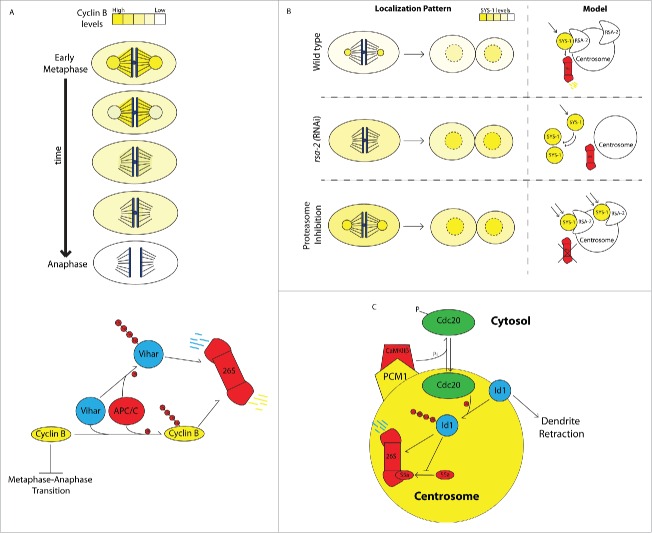
Figure 2.
Centrosome-associated degradation pathways. A) Centrosome and spindle-associated degradation of Cyclin B. Top panel: At metaphase, degradation of cyclin B begins at centrosomes in a step-wise pattern that propagates to the mitotic spindle in a direction toward the spindle equator and is effectively cleared prior to anaphase entry. Bottom panel: Centrosome-bound cyclin B is polyubiquitinated (small red circles represent ubiquitin tags) by the E3 ubiquitin ligase APC/C and the E2 ubiquitin conjugating enzyme Vihar, marking it for degradation by the proteasome. Vihar is polyubiquitinated and marked for degradation by APC/C, resulting in a negative feedback loop on APC/C ubiquitination of cyclin B. (B) Centrosome-associated degradation of SYS-1 during C. elegans asymmetric cell division. Top panel: SYS-1 localizes to centrosomes, where it is subject to dynamic turnover via the proteasome during division and is asymmetrically distributed between daughter cell nuclei after division. Middle panel: When SYS-1 centrosomal localization is disrupted by rsa-2 (RNAi), its degradation is attenuated during division, resulting in elevated SYS-1 levels in both daughter cell nuclei. Bottom panel: When the proteasome is inhibited, SYS-1 accumulation at centrosomes is increased and its turnover is decreased. In the absence of the proteasome, SYS-1 protein avoids negative regulation during asymmetric division and is severely overaccumulated in both daughter cells. (C) Centrosomal degradation pathways control dendrite growth in neurons. CaMKIIβ104 binds centrosomes by interaction with PCM1 and phosphorylates the APC/C component Cdc20, causing it to dissociate from centrosomes. Unphosphorylated Cdc20 localizes to centrosomes and polyubiquitinates the helix-loop-helix protein Id1 targeting it for degradation by the centrosomal proteasome. In the absence of negative regulation by Cdc20 and the proteasome, Id1 retracts dendrites. Id1 negatively regulates proteasomes by preventing association of S5a with the 19S complex, potentially autoregulating its own degradation.