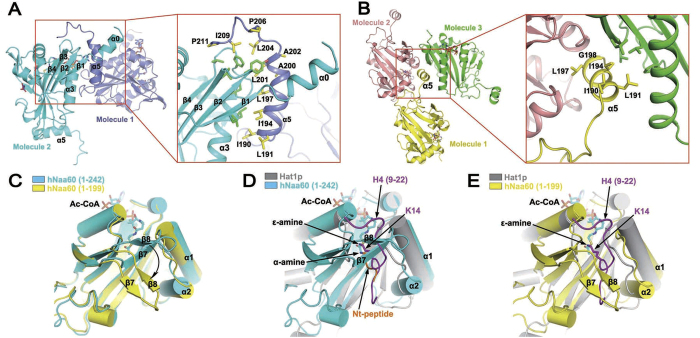
Figure 2. Amphipathicity of the α5 helix and alternative conformations of the β7-β8 hairpin.
(A) The α5 helix of hNaa60(1-242) in one asymmetric unit (slate) interacts with another hNaa60 molecule in a neighboring asymmetric unit (cyan). A close view of the interaction is shown in red box. Side-chains of hydrophobic residues on α5 helix and the neighboring molecule participating in the interaction are shown as yellow and green sticks, respectively. (B) The α5 helix of hNaa60(1-199) in one asymmetric unit (yellow) interacts with another hNaa60 molecule in the neighboring asymmetric units (green). A close view of the interaction is shown in the red box. Side-chains of hydrophobic residues on α5 helix and the neighboring molecule (green) participating in the interaction are shown as yellow and green sticks, respectively. The third molecule (pink) does not directly interact with the α5 helix. (C) Superposition of hNaa60(1-199) (yellow) and hNaa60(1-242) (cyan) showing conformational change of the β7-β8 hairpin in these two structures. (D,E) Superposition of Hat1p/H4 (gray, drawn from PDB 4PSW) with hNaa60(1-242) (cyan, D) or hNaa60(1-199) (yellow, E). The histone H4 peptide (a KAT substrate) bound to Hat1p is shown in purple (D,E), while the peptide bound to hNaa50 (a NAT substrate, drawn from PDB 3TFY) is shown in orange (Nt-peptide) after superimposing hNaa50 (not shown in figure) on hNaa60 (D). The α-amine of the NAT substrate and ε-amine of the KAT substrate (along with the lysine side-chain) subject to acetylation are shown as sticks.