Figure 6. Model of mechanism of lysine transport in S. cerevisiae.
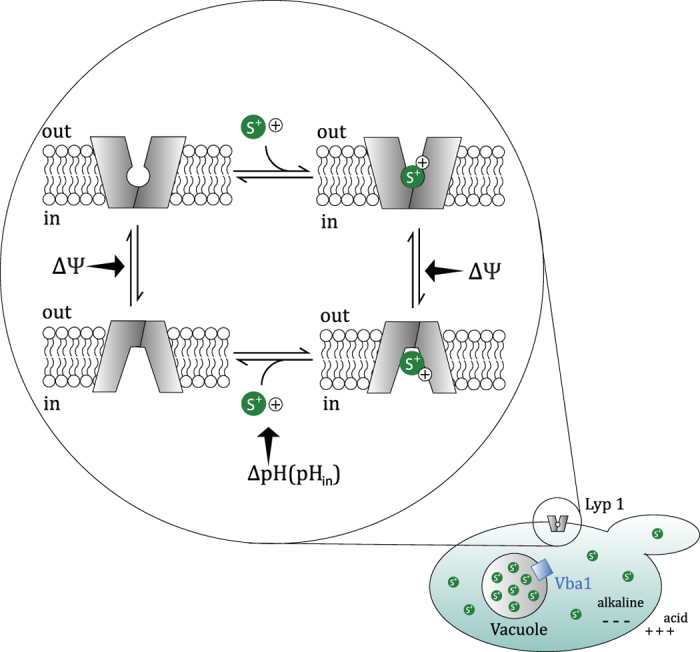
In the cell a fraction of the lysine is accumulated in the vacuole, mostly due to Vba1-mediated uptake, which reduces the effective concentration of lysine in the cytoplasm. The in-to-out Michaelis constant (KM) of Lyp1 for lysine transport is very high (depicted by a different inward-facing conformation) as compared to the out-to-in KM (more tight pocket for lysine, S+). The import rate increases with the membrane potential (ΔΨ), and most likely the ΔΨ accelerates the out-to-in or in-to-out isomerization of the protein (indicated by arrows). A pH gradient (ΔpH) increases the driving force, resulting in higher accumulation levels, but also increases the rate of transport by Lyp1. Here, the ΔpH (increased internal pH) may facilitate the release of the proton (+) when the protein is in the inward-facing conformation.
