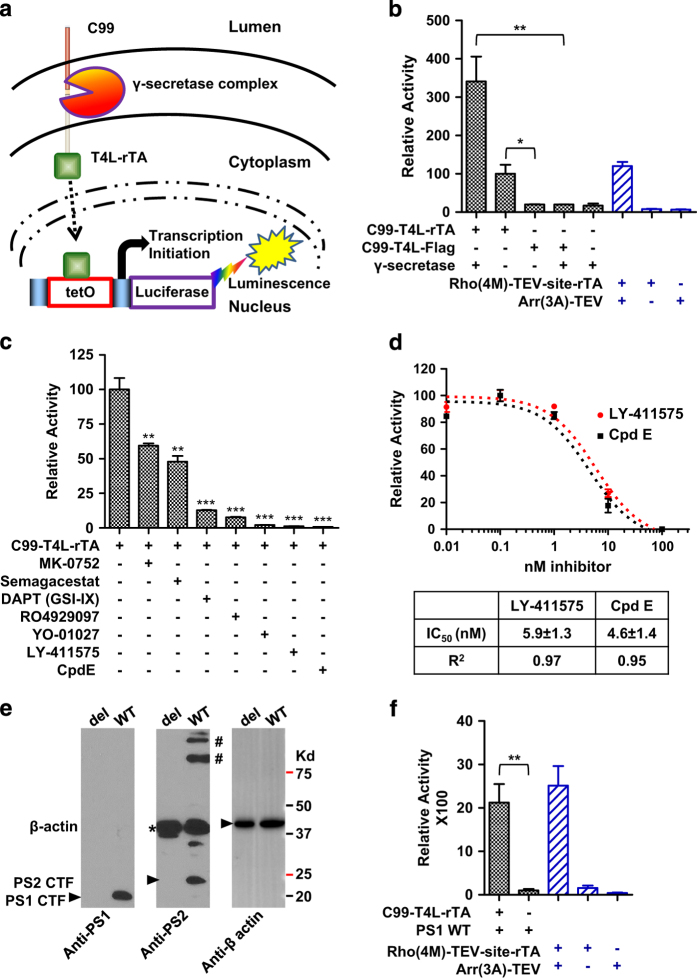
Figure 1.
Development and validation of the γ-secretase epsilon-cleavage assay to quantitatively determine relative C99 cleavage by γ-secretase in cells. (a) Schematic overview of the γ-secretase epsilon-cleavage assay. Upon membrane cleavage of the C99 hybrid protein by γ-secretase, Aβ peptides are released into the medium and the AICD-T4L-rTA hybrid protein is released from the membrane into the cytoplasm. This allows the hybrid protein to enter the nucleus and to bind tetO DNA-binding site to stimulate luciferase reporter gene activity as measurement for total C99 cleavage, both by endogenous and by transfected γ-secretase variants. (b) Relative reporter gene activity using C99-T4L-rTA. Rho(4M)-TEV-site-rTA and Arr(3A)-TEV serve as positive control using a conventional Tango protein–protein interaction assay. (c) Cleavage of the C99 hybrid substrate is affected by γ-secretase inhibitors (100 nm per well), indicating that cleavage in HTL cells is due to endogenous γ-secretase. (d) IC50 dose–response curves for the two most potent γ-secretase inhibitors (LY-411575 compound and compound E (Cpd E)). (e) Immunoblot validation of CRISPR/Cas9-mediated chromosomal PS1 and PS2 deletions. PS1 and PS2 protein levels of wildtype (WT) HTL and double-deletion cells are determined by immunoblotting using antibodies that detect the PS1 C-terminal fragment (CTF) (Cell Signaling Technologies 3622S) and antibodies that detect PS2 CFT (Abcam ab106351). β-actin antibody (Abcam ab6276) is used for normalization. *Antibody cross-reactive band. #PS2 membrane protein oligomers. (f) Chromosomal deletion of PS1 and PS2 abolishes reporter gene activity. Activity can be restored by transfecting WT PS1 gene to a similar level as the positive control of Rho(4M)-TEV-site-rTA and Arrestin(3A)-TEV. (error bars=s.e.m., n=3, P-values (two-tailed Student’s t-test versus control (a, f) or WT (c)): *P<0.05; **P<0.01; ***P<0.001).