Figure 1.
Pathogenic models of retinal damage in NMOSD–ON eyes.
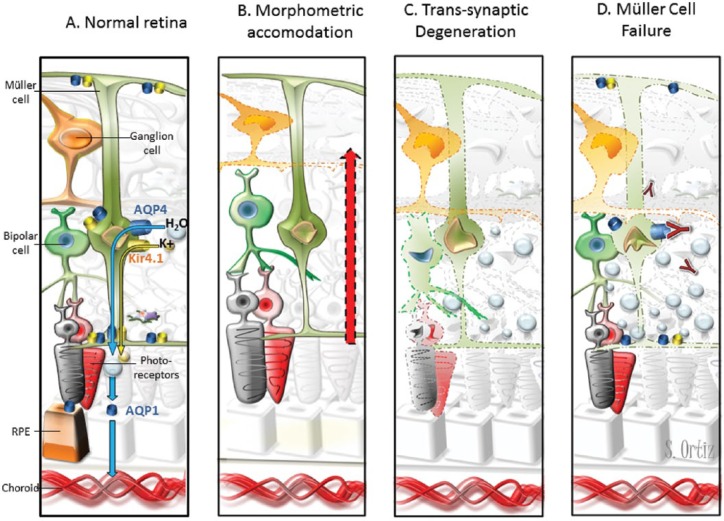
(A) Water transportation through the retina. Extracellular fluid accumulates in the neural retina and subretinal space. Müller cells and RPE cells promote fluid clearance towards the vessels using aquaporins receptors. Müller cell express AQP4, and RPE cells express AQP1. Moreover, water transportation is coupled to the transport of potassium through Kir4.1 channels. Müller cells display AQP4 and Kir4.1 surrounding the vessels of INL and at both limiting membranes. (B) The hypothesis of morphometric accommodation. Müller cells may promote thickening of other layers as compensatory effect to mGCC thinning after AON. We would expect proportional or greater thickening in adjacent layers (INL/OPL) and we found thickening of INL and ONL but OPL thinning. (C) The hypothesis of trans-synaptic degeneration. Retrograde degeneration of ganglion cells may induce trans-synaptic degeneration that finally lead to neuronal loss in the retina. Neuronal loss would translate in layer thinning instead of thickening. However, trans-synaptic degeneration may promote Müller cell dysfunction with fluid accumulation leading to retinal thickening that mask neuronal loss. (D) The hypothesis of Müller cell dysfunction. Inflammation in the acute phase of optic neuritis may induce Kir4.1 down regulation leading to water accumulation and transient thickening of outer layers [Gabilondo et al. 2015]. In patients with AQP4-antibodies, the immune-mediated damage may promote Müller cells loss. This would likely produce water accumulation in the neural retina but not in the subretinal space because RPE cells express AQP1. In our study, NMOSD–ON eyes patients with AQP4-IgG displayed INL and ONL thickening but not RPE thickening.
AQP, aquaporin; INL, inner nuclear layer; NMOSD, neuromyelitis optica spectrum disorder; ON, optic neuritis; RPE, retinal pigment epithelium; AON, acute optic neuritis; mGCC, macular ganglion cell complex; ONL, outer nuclear layer; OPL, outer plexiform layer.