Abstract
Background:
Postoperative infection is a devastating complication following arthroplasty. The goals of this study were to introduce a “smart” implant coating that combines passive elution of antibiotic with an active-release mechanism that “targets” bacteria, and to use an established in vivo mouse model of post-arthroplasty infection to longitudinally evaluate the efficacy of this polymer implant coating in decreasing bacterial burden.
Methods:
A novel, biodegradable coating using branched poly(ethylene glycol)-poly(propylene sulfide) (PEG-PPS) polymer was designed to deliver antibiotics both passively and actively. In vitro-release kinetics were studied using high-performance liquid chromatography (HPLC) quantification in conditions representing both the physiologic environment and the more oxidative, hyperinflammatory environment of periprosthetic infection. The in vivo efficacy of the PEG-PPS coating delivering vancomycin and tigecycline was tested using an established mouse model of post-arthroplasty infection. Noninvasive bioluminescence imaging was used to quantify the bacterial burden; radiography, to assess osseointegration and bone resorption; and implant sonication, for colony counts.
Results:
In vitro-release kinetics confirmed passive elution above the minimum inhibitory concentration (MIC). A rapid release of antibiotic was noted when challenged with an oxidative environment (p < 0.05), confirming a “smart” active-release mechanism. The PEG-PPS coating with tigecycline significantly lowered the infection burden on all days, whereas PEG-PPS-vancomycin decreased infection on postoperative day (POD) 1, 3, 5, and 7 (p < 0.05). A mean of 0, 9, and 2.6 × 102 colony-forming units (CFUs) grew on culture from the implants treated with tigecycline, vancomycin, and PEG-PPS alone, respectively, and a mean of 1.2 × 102, 4.3 × 103, and 5.9 × 104 CFUs, respectively, on culture of the surrounding tissue (p < 0.05).
Conclusions:
The PEG-PPS coating provides a promising approach to preventing periprosthetic infection. This polymer is novel in that it combines both passive and active antibiotic-release mechanisms. The tigecycline-based coating outperformed the vancomycin-based coating in this study.
Clinical Relevance:
PEG-PPS polymer provides a controlled, “smart” local delivery of antibiotics that could be used to prevent postoperative implant-related infections.
Periprosthetic joint infection remains a devastating complication of total joint arthroplasty. Despite advances in sterile surgical technique and the use of perioperative antibiotics, periprosthetic joint infection occurs in 1% of primary and 3% to 7% of revision total joint arthroplasties1-7. It leads to increased patient disability, morbidity, and even mortality. Patients with periprosthetic joint infection often require prolonged intravenous antibiotics and multiple surgeries and often end up with diminished functional use of the extremity8,9.
The concept of the local delivery of antimicrobial therapy to maximize efficacy while minimizing systemic effects has been available for decades in the form of solutions, ointments, and creams. In arthroplasty, local antimicrobial strategies have ranged from adding antibiotics to irrigation fluid to the use of vancomycin powder in the wound prior to closure10,11. However, the presence of antibiotic levels in the soft tissue around the implant is short-lived, and these approaches fail to create a protective barrier on the implant that prevents bacterial colonization and subsequent biofilm formation12. The concept of antimicrobial implant coatings has been suggested as a method to remedy these concerns. Currently, antimicrobial coatings are clinically available as antibiotic-impregnated cement (polymethylmethacrylate [PMMA]) in the U.S., iodine coating in Japan, and nanosilver coating in Europe13. While these coatings have shown promise, all 3 have poorly controlled release kinetics. Additionally, PMMA is an inert, permanent vehicle (providing an additional surface for colonization), and both iodine and nanosilver have questionable toxicity profiles involving the release of halogen or metallic ions in the bloodstream, respectively. All 3 act via passive release of antimicrobial agents such that there is a nonspecific local effect initiated on implantation, independent of the presence or absence of bacteria.
The aim of the current study was to devise a novel, nontoxic, biodegradable poly(ethylene glycol)-poly(propylene sulfide) (PEG-PPS) polymer coating that can be used as a vehicle to deliver antibiotics locally through both a passive and active mechanism. The active release is driven by the reactive oxygen cascade initiated by the presence of bacteria, allowing the “smart” polymer to release antibiotic where it is needed most. This coating therefore targets bacteria-rich environments and diffuses the antibiotic down a gradient toward the bacterial challenge. Because the polymer is completely biodegradable, no additional foreign materials are retained in the body once the antibiotic is eluted from the implant. On the basis of previous work, we used vancomycin and tigecycline as the antibiotics of choice in this study, as they showed optimal efficacy against Staphylococcus aureus, the causative organism in nearly half of arthroplasty infections14-21. We endeavored to examine the in vitro release properties of this novel coating, and used an established in vivo mouse model of post-arthroplasty infection to test the efficacy of the coating as a vehicle for the delivery of antibiotics to eradicate infection and prevent biofilm formation.
Materials and Methods
Synthesis of PEG-PPS Polymer and Coating of the Implant
The synthesis of PEG-PPS polymer follows a 3-step chemical reaction. First, 4-armed PEG (10 g), molecular weight (MW) 20,000, was dissolved in dried tetrahydrofuran (THF) and refluxed under argon gas at 90°C for 4 hours. After cooling, sodium hydride (0.6 g) was slowly added, and the mixture was stirred for 15 minutes under argon. Subsequently, allyl bromide (1.6 mL) was added to the mixture, and the reaction was stirred overnight under argon gas. Second, PEG-allyl (3.78 g) was combined with anhydrous toluene in a Schlenk tube. The radical initiator 2,2’-azobis(2-methylpropionitrile) (AIBN) (1.5 g) was freshly prepared via crystallization in methanol and added to the PEG-allyl and toluene. The product of PEG-thiol acetate had a 93% yield and was confirmed with nuclear magnetic resonance (NMR) spectroscopy in CDCl3 to have all allyl groups fully converted. Finally, sodium methoxide (82 mg) was added to the PEG-thiol acetate and THF under argon and stirred for 30 minutes at room temperature. Subsequently, PPS (5× molar equivalent of PEG-PPS arms) was added under argon, and the reaction mixture was stirred for 1 hour. The end-capping reagent 2,2′-dithioldipyridine (10× molar equivalent) was later added, and the reaction mixture was stirred under argon overnight. The mixture was later dried via a rotary evaporator and dialyzed extensively against water in regenerated cellulose dialysis tubing (molecular weight cut-off of 3,000 to 6,000). Lastly, the product of PEG-PPS was lyophilized and stored under argon at −20°C.
Titanium Kirschner wires with a surface area of 44.2 mm2 (diameter, 0.8 mm; length, 8 mm) underwent oxygen plasma treatment at 200 mTorr, 200 W for 15 minutes. Subsequently 1% (v/v) (3-mercaptopropyl) trimethoxysilane was reacted with the Kirschner wires in toluene at 90°C with stirring, followed by sonication in chloroform (5 times), acetone (2 times), methanol (5 times), and in Milli-Q water (Millipore) (5 times). The Kirschner wires were then heated at 50°C for at least 30 minutes until dry. The PEG-PPS polymer was then dissolved in phosphate-buffered saline (PBS) solution to make either a 3% or 6% (w/v) solution. Three hundred microliters of PEG-PPS solution with dissolved tigecycline or vancomycin at 20 mg/mL was used to coat a batch of 6 to 8 of the treated Kirschner wires for in vivo study. The Kirschner wires were submerged in the antibiotic-encapsulated PEG-PPS solution or PEG-PPS solution alone at 4°C and dried at 50°C. This wet-dry cycle was repeated a total of 10 times. After the coating cycles, the remainder of the coating solution (approximately a quarter of the original) was discarded. To aid in visualization of the coating, rhodamine was also coated on the titanium pins via encapsulation in the PEG-PPS solution. Additionally, the PEG-PPS coating was tagged with near-infrared (NIR) dye so that the elution of the coating could be measured noninvasively in vivo. The microstructure and surface compositional changes of the implant resulting from the polymer coating were examined using a scanning electron microscope (Nova NanoSEM 230; FEI) and energy dispersive spectroscopy (EDS). Representative images were acquired under standard operation, and EDS analysis was performed under 10.0 kV voltage and at a 35.3° take-off angle.
In Vitro Release Kinetics
In vitro release of the coated antibiotics (coating solution at 10 mg of antibiotic/mL of PEG-PPS solution) was conducted by submerging the Kirschner wires in 150 μL of PBS solution and keeping them at 37°C. As a control, PEG-PPS-coated pins without antibiotic were also assayed for release in PBS solution, as the coating is bioresorbable in aqueous solution. The buffer was refreshed daily for at least 1 week, and the amount of released vancomycin and tigecycline was quantified by high-performance liquid chromatography (HPLC) based on their ultraviolet absorption at 280 or 245 nm, respectively, using 0.1% trifluoroacetic acid as the flowing phase at a flow rate of 0.1 mL/min.
In Vivo Assessment of Coating
All procedures were approved by the institution’s animal research committee. The bioluminescent S. aureus Xen36 strain contains a bioluminescent lux operon construct integrated into a stable bacterial plasmid that naturally produces a blue-green light emitted only by metabolically active bacteria22. Xen36 previously was demonstrated to be optimal for use with the established mouse model of post-arthroplasty infection selected for the current study and was grown and cultured as previously described23-26. Twelve-week-old, male C57BL/6 wild-type mice (Jackson Laboratory) were used in all experiments. To model an orthopaedic implant infection, a medical-grade, 0.8-mm-diameter titanium Kirschner-wire implant, precoated with PEG-PPS, PEG-PPS encapsulating vancomycin, or PEG-PPS encapsulating tigecycline, was surgically placed into the distal aspect of the right femur of the mice, and the joint was challenged with Xen36, as previously described23-26. Sustained-release buprenorphine (2.5 mg/kg) (Zoopharm) was administered at the time of surgery and every 3 days postoperatively. Mice were anesthetized via inhalation of isoflurane (2%), and in vivo bioluminescence imaging was performed by using the IVIS Lumina II in vivo imaging system (PerkinElmer), as previously described23-27. The bioluminescence signals were measured on postoperative day (POD) 0, 1, 3, 5, 7, 10, 14, and 21. To confirm that the bioluminescence signals corresponded to the bacterial burden in vivo, bacteria adherent to the implants and surrounding tissue were quantified through sonication and colony-forming unit (CFU) counting, as previously described23-27. High-resolution radiographs were made on POD 0, 7, 14, 21, and 28 to qualitatively assess osseointegration and bone resorption. All radiographs were made using a Quados Faxitron LX-60 Cabinet radiography system with a variable kV point projection x-ray source and digital imaging system (Cross Technologies). Finally, to confirm that the coating was completely resorbed by 14 days, NIR imaging was performed on POD 0, 3, 7, and 14.
Statistical Analysis
Data for multiple comparisons were analyzed using a one-way ANOVA (analysis of variance) followed by the Tukey test, and single comparisons were analyzed using a Student t test (two-tailed). All data are expressed as the mean and the standard error of the mean (SEM), where indicated. Values of p < 0.05 were considered significant.
Results
PEG-PPS Implant Coating and In Vitro Release Kinetics
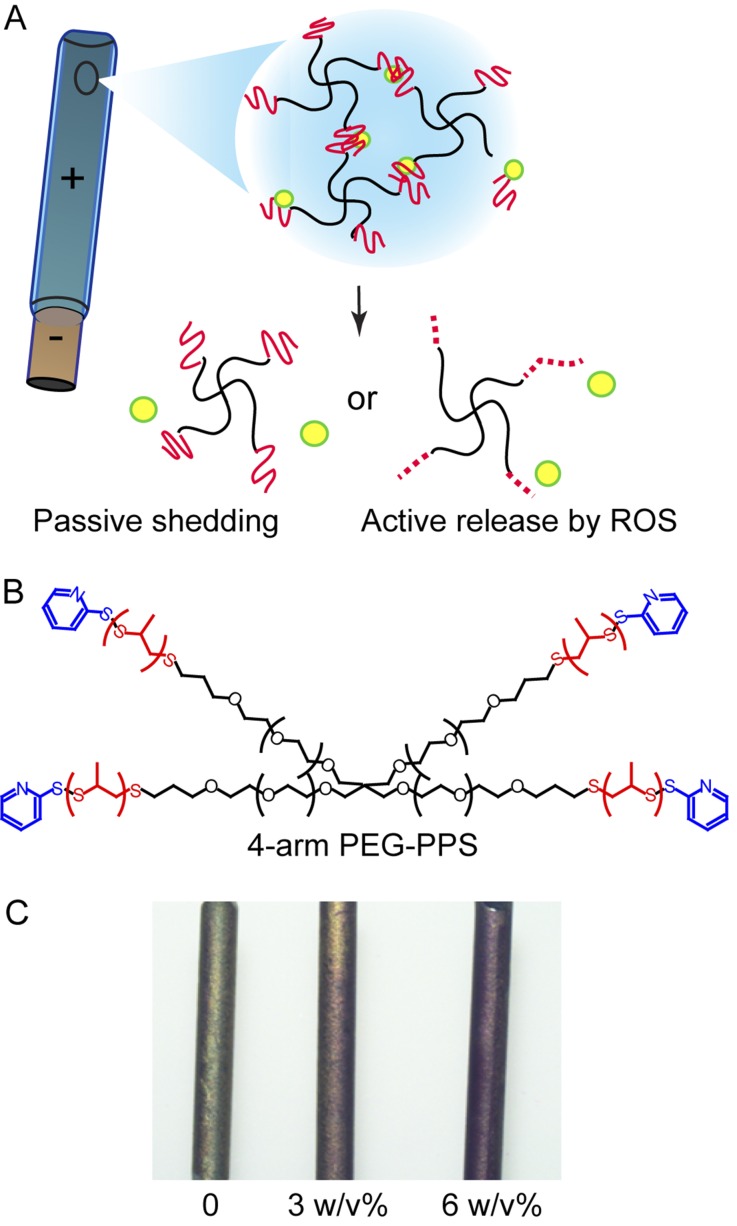
The PEG-PPS coating consisted of a 4-arm molecule with the ability to both passively shed antibiotic as well as actively release it in a highly oxidative environment (Figs. 1-A and 1-B). The coating was evaluated using both visualization through the model molecule rhodamine and scanning electron microscopy, which confirmed a uniform coating on the surface of the titanium Kirschner wire (Fig. 1-C).
Fig. 1.
Schematics of the 4-armed PEG-PPS polymer coating on a metal implant for passive elution or active release of antibiotics by reactive oxygen species (ROS) (Figs. 1-A and 1-B) and visualization of the polymer coating through use of a model molecule, rhodamine (Fig. 1-C; left, bare titanium wire with no coating; middle, rhodamine encapsulated in 3% (w/v) PEG-PPS coating on a titanium wire; right, rhodamine in 6% (w/v) PEG-PPS on a titanium wire).
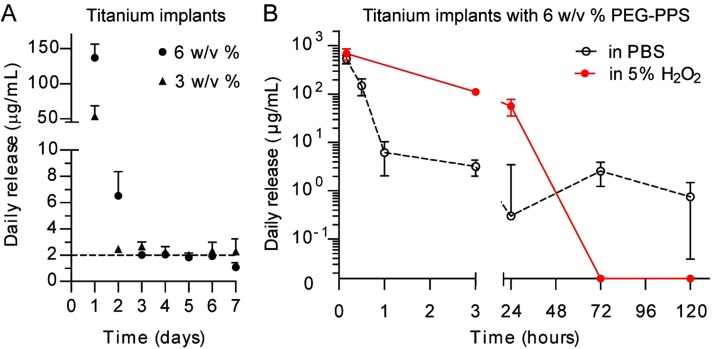
As the polymer concentration increased from 3% to 6% (w/v), the amount of releasable antibiotic payload increased (Fig. 2-A), cumulatively, to 10.2 ± 4.1 μg for 7 days per pin coated in 3% (w/v) PEG-PPS and 22.8 ± 5.4 μg for 7 days per pin coated in 6% (w/v) PEG-PPS. The daily release of vancomycin and tigecycline from the PEG-PPS-coated titanium was consistent and above the minimum inhibitory concentration (MIC) (2 μg/mL) for 7 days for the 3% (w/v) and 4 days for the 6% (w/v) coatings.
Fig. 2.
Figs. 2-A and 2-B In vitro passive and active release of antibiotic from the PEG-PPS polymer (mean and SEM; n = 3). Fig. 2-A The mass of cumulatively released vancomycin over days per pin showed that 6% (w/v) PEG-PPS had a higher loading efficiency. Fig. 2-B Daily release of vancomycin per pin, quantified via HPLC, showed a “burst” release when challenged by an oxidative environment representative of a bacterial infection compared with a sustained release in a nonoxidative environment.
The release of payload antibiotics from the PEG-PPS coating was enhanced in the presence of reactive oxygen species (ROS), as would occur in the presence of a periprosthetic joint infection. The vancomycin-loaded PEG-PPS coatings in PBS solution slowly released vancomycin from POD 1 to 5, whereas identical coatings in 5% (v/v) H2O2 solution rapidly released all vancomycin within 3 days (p < 0.05) (Fig. 2-B). This result is indicative of the “smart” polymer design to actively release payload in an oxidative environment.
In Vivo Efficacy of the Antibiotic-Loaded PEG-PPS Coatings
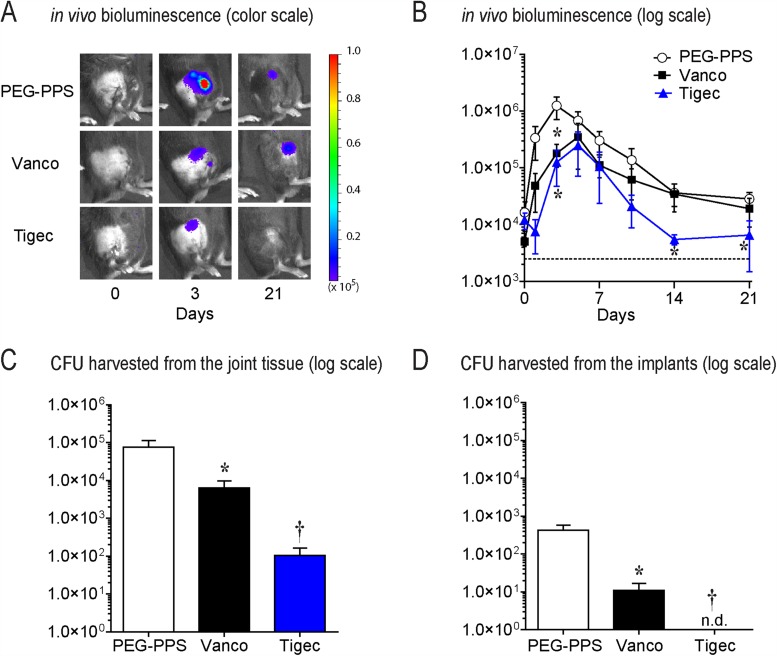
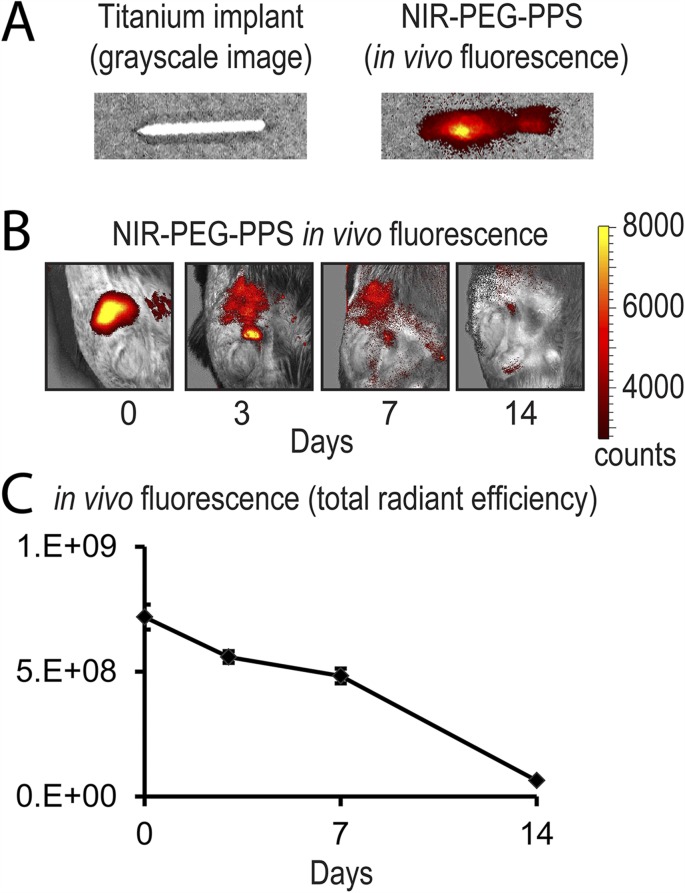
The bioluminescent signals were significantly lower (p < 0.05) in the mice possessing the vancomycin implants (POD 1, 3, 5, and 7) and tigecycline implants (all time points evaluated) compared with those in the mice with implants treated with the PEG-PPS coating alone (Figs. 3-A and 3-B). The tigecycline-coated implants had a bioluminescence signal that was not above baseline from POD 14 onward, suggesting eradication of infection below the level of detection by noninvasive imaging. The quantification of CFUs from the implants and surrounding tissue on POD 21 showed a decreased number of CFUs cultured from both the vancomycin and tigecycline groups compared with the PEG-PPS group control (Figs. 3-C and 3-D). While all implants from the PEG-PPS group grew out bacteria (mean, 2.6 × 102 CFUs), only 60% of the implants in the vancomycin group (mean, 9 CFUs) and 0% of the implants in the tigecycline group had bacterial growth (p < 0.05). In the surrounding bone and joint tissue, all specimens in both the PEG-PPS and vancomycin groups had CFU growth (mean, 5.9 × 104 and 4.3 × 103 CFUs, respectively), whereas only 60% of the tigecycline group had any CFUs isolated in the surrounding tissue (mean, 1.2 × 102 CFUs) (p < 0.05). In summary, a significant reduction of CFUs from the implants and the surrounding bone and joint tissue was observed in both the vancomycin and tigecycline implant groups. However, the tigecycline-coated implants were the most effective, as the bacteria were cleared from implants in all cases, and CFUs were present in only 60% of the surrounding bone and joint tissue. Finally, as a confirmation of safety and biodegradability of the PEG-PPS polymer, NIR imaging showed a complete resorption of the polymer by POD 14 (Fig. 4).
Fig. 3.
Figs. 3-A through 3-D In vivo efficacy of the PEG-PPS coating in decreasing bacterial infection. Vanco = vancomycin, and tigec = tigecycline. Error bars indicate the SEM. Fig. 3-A Representative in vivo S. aureus bioluminescence on a color scale overlaid on grayscale images of mice. Fig. 3-B Postoperative in vivo S. aureus bioluminescence signals (mean maximum flux [photons/sec/cm2/sr] and SEM) (logarithmic scale). *P < 0.01. The dashed line is the sensitivity limit of the assay for light. Fig. 3-C Quantification of colony-forming units (CFUs) cultured from surrounding tissue on POD 21. *P = 0.01; †p < 0.05. Fig. 3-D Quantification of CFUs cultured from implants on POD 21. N.d. = none detected. *P = 0.01; †p < 0.05.
Fig. 4.
Figs. 4-A, 4-B, and 4-C In vivo biodegradation of the coating. Fig. 4-A Representative in vivo fluorescence on a color scale overlaid on a grayscale image of a titanium implant (left) versus titanium implant coated with PEG-PPS embedded with a near-infrared (NIR) dye. Fig. 4-B Representative in vivo fluorescence showing a diminishing NIR signal over 14 days from baseline, demonstrating complete biodegradation of the coating. Fig. 4-C Postoperative in vivo fluorescence signals (mean total radiant efficiency [(photons/sec)/(μH/cm2)] and SEM) (logarithmic scale) quantifying this biodegradation.
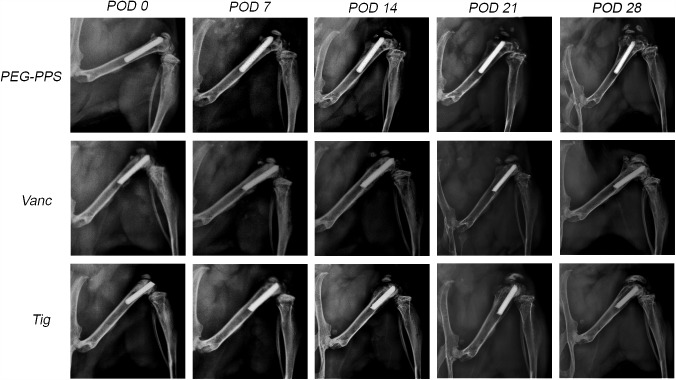
Implants coated with PEG-PPS alone showed a dramatic degree of periprosthetic osteolysis that became evident by POD 7 and progressed over time. In contrast, antibiotic-encapsulated PEG-PPS implants showed no detectable radiographic periprosthetic osteolysis (Fig. 5), consistent with the high efficacy of these coatings in facilitating bacterial clearance from the implants.
Fig. 5.
Representative radiographs from the PEG-PPS, vancomycin (Vanc), and tigecycline (Tig) groups from POD 0 to 28. There was an observable increase in condylar osteolysis and reactive bone formation in the PEG-PPS group compared with the tigecycline and vancomycin groups, which showed no detectable radiographic evidence of osteolysis. This suggests clinical relevance of the decrease in bacterial burden in the antibiotic-encapsulated coatings.
Discussion
Infection after total joint arthroplasty represents a clinically devastating complication that is exceedingly difficult to prevent and treat9,28,29. The difficulty is due to several host and pathogen factors, including a high affinity of bacteria for the foreign implant surface; the formation of biofilm, which blocks the penetration of immune cells and systemic antibiotics; and the emergence of antibiotic-resistant organisms8,9,28-36. Given this, novel strategies are needed to minimize the number of such cases that develop infection.
The present study demonstrated that antibiotics—in this case, vancomycin and tigecycline—can be loaded into a PEG-PPS polymer coating covalently linked to metal implants. These antibiotics can then be passively released over the course of 1 week to maintain therapeutic levels during the perioperative period, or actively induced to release antibiotic more rapidly in the face of a bacterial challenge. This is in stark contrast to the poorly controlled, erratic antibiotic release from PMMA37. The local release of vancomycin and tigecycline from the PEG-PPS coatings resulted in a significantly lower bacterial burden as measured by in vivo bioluminescence imaging, with confirmatory quantification of CFUs isolated from the implants and surrounding bone and joint tissue on POD 21. Interestingly, the tigecycline-loaded PEG-PPS implants achieved a more potent antibacterial effect than did the vancomycin-loaded PEG-PPS and prevented bacterial colonization on 100% of the implants.
The PEG-PPS coating is unique, as it consists of an inner layer of polymer covalently linked to the implant and self-assembled outer layers that allow for an active release. This release, triggered by the bacterially induced hyperinflammatory state, can drive the diffusion of antibiotic. These unique properties have the potential to protect the implant from bacterial adherence and biofilm formation as can occur through direct contamination intraoperatively, or seeding of the implant from transient bacteremia or infected hematoma formation during the acute postoperative period1,9,28,29,34-36.
In our study, the tigecycline-encapsulated PEG-PPS coating was particularly effective in preventing any colonization of bacteria on the implant surface (as evidenced by 0 CFUs cultured from the implant) as well as significantly reducing the CFUs isolated from the surrounding bone and joint tissue. The enhanced efficacy of tigecycline versus vancomycin-coated implants requires further investigation. However, a possible explanation for the increased efficacy is that tigecycline has preferential uptake in rodent and human bone compared with vancomycin38-40. Additional studies should evaluate the efficacy of these antibiotic polymer coatings at higher concentrations, of combinations of antibiotics (such as the synergistic effect of adding rifampin, which has enhanced therapeutic effect against periprosthetic joint infection in cases of implant retention41-44), or of these antibiotic polymers in combination with intravenous prophylactic perioperative antibiotics.
An important limitation of this coating is that it is designed to be short-acting and biodegradable and, therefore, will only be effective in preventing infection seeded during surgery or in the immediate postoperative period. It will not be present for sources of infection due to hematogenous spread occurring after the postoperative period at any point during the lifetime of the implant. Its absorption is presumed to be advantageous, as it does not impact osseointegration or provide an additional surface for bacterial adherence, which are important for the long-term biocompatibility of the implant. Additionally, this coating was designed to completely elute from the implant by 14 days. As the coating is made by a layering technique, a longer-duration PEG-PPS coating is easily achievable by adding more layers.
There were several limitations of the study. First, only the efficacy against S. aureus was evaluated. Additional studies should include other organisms, such as S. epidermidis, Propionibacterium acnes, and methicillin-resistant S. aureus (MRSA). Additionally, while small-animal models inherently simplify the surgical procedure, lessen the cost of experimentation, provide options for immunomodulation, and enable rapid feedback for improvements, the findings from such studies cannot be assumed to be the same as those in larger animals or humans. Further testing in large animals and humans is needed. This subsequent work could also better investigate the host immune response to such infections45, an avenue of study that was beyond the scope of this project.
Despite these limitations, we believe that this novel PEG-PPS implant coating is an effective tool to deliver various antibiotics or even combinations of antibiotics locally during the perioperative period. This coating is versatile in that it can be loaded with many different antibiotics or antimicrobials, has a passive-release mechanism to consistently ensure levels above the desired MIC to prevent the development of antibiotic resistance, and has an active-release mechanism that responds to the presence of bacteria with increased antibiotic release. Additionally, the coating is completely biodegradable and can be easily applied to implants of all shapes and sizes. In summary, “smart” antimicrobial implant coatings, such as the PEG-PPS coating described in this study, have great potential to minimize the incidence of postoperative infection following arthroplasty.
Footnotes
Investigation performed at the University of California, Los Angeles, Los Angeles, California
Disclosure: This research was supported by the NIH/National Center for Advancing Translational Science (NCATS) UCLA CTSI Grant Number KL2TR000122 (N.M.B.), the Orthopaedic Research and Education Foundation (OREF) Young Investigator Award (N.M.B.), the AO Foundation Start-up Grant Program (L.S.M. and J.A.N.), and an H & H Lee Surgical Resident Research Scholars Program Grant (A.I.S.). On the Disclosure of Potential Conflicts of Interest forms, which are provided with the online version of the article, one or more of the authors checked “yes” to indicate that the author had a patent and/or copyright, planned, pending, or issued, broadly relevant to this work.
References
- 1.Illingworth KD, Mihalko WM, Parvizi J, Sculco T, McArthur B, el Bitar Y, Saleh KJ. How to minimize infection and thereby maximize patient outcomes in total joint arthroplasty: a multicenter approach: AAOS exhibit selection. J Bone Joint Surg Am. 2013. April 17;95(8):e50. [DOI] [PubMed] [Google Scholar]
- 2.Lentino JR. Prosthetic joint infections: bane of orthopedists, challenge for infectious disease specialists. Clin Infect Dis. 2003. May 1;36(9):1157-61. Epub 2003 Apr 14. [DOI] [PubMed] [Google Scholar]
- 3.Best JT. Revision total hip and total knee arthroplasty. Orthop Nurs. 2005. May-Jun;24(3):174-9; quiz 180-1. [DOI] [PubMed] [Google Scholar]
- 4.Blom AW, Brown J, Taylor AH, Pattison G, Whitehouse S, Bannister GC. Infection after total knee arthroplasty. J Bone Joint Surg Br. 2004. July;86(5):688-91. [DOI] [PubMed] [Google Scholar]
- 5.Bozic KJ, Kurtz SM, Lau E, Ong K, Vail TP, Berry DJ. The epidemiology of revision total hip arthroplasty in the United States. J Bone Joint Surg Am. 2009. January;91(1):128-33. [DOI] [PubMed] [Google Scholar]
- 6.Jafari SM, Coyle C, Mortazavi SM, Sharkey PF, Parvizi J. Revision hip arthroplasty: infection is the most common cause of failure. Clin Orthop Relat Res. 2010. August;468(8):2046-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007. April;89(4):780-5. [DOI] [PubMed] [Google Scholar]
- 8.Del Pozo JL, Patel R. Clinical practice. Infection associated with prosthetic joints. N Engl J Med. 2009. August 20;361(8):787-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zimmerli W, Trampuz A, Ochsner PE. Prosthetic-joint infections. N Engl J Med. 2004. October 14;351(16):1645-54. [DOI] [PubMed] [Google Scholar]
- 10.Kruckenhauser EM, Nogler M, Coraça-Huber D. Use of lavage fluids in arthroplasty to prevent postoperative infections. Drug Res (Stuttg). 2014. March;64(3):166-8. Epub 2013 Aug 28. [DOI] [PubMed] [Google Scholar]
- 11.Qadir R, Ochsner JL, Chimento GF, Meyer MS, Waddell B, Zavatsky JM. Establishing a role for vancomycin powder application for prosthetic joint infection prevention-results of a wear simulation study. J Arthroplasty. 2014. July;29(7):1449-56. Epub 2014 Feb 12. [DOI] [PubMed] [Google Scholar]
- 12.Alijanipour P, Heller S, Parvizi J. Prevention of periprosthetic joint infection: what are the effective strategies? J Knee Surg. 2014. August;27(4):251-8. Epub 2014 May 3. [DOI] [PubMed] [Google Scholar]
- 13.Shirai T, Tsuchiya H, Nishida H, Yamamoto N, Watanabe K, Nakase J, Terauchi R, Arai Y, Fujiwara H, Kubo T. Antimicrobial megaprostheses supported with iodine. J Biomater Appl. 2014. October;29(4):617-23. Epub 2014 Jun 9. [DOI] [PubMed] [Google Scholar]
- 14.Fulkerson E, Valle CJ, Wise B, Walsh M, Preston C, Di Cesare PE. Antibiotic susceptibility of bacteria infecting total joint arthroplasty sites. J Bone Joint Surg Am. 2006. June;88(6):1231-7. [DOI] [PubMed] [Google Scholar]
- 15.Salgado CD, Dash S, Cantey JR, Marculescu CE. Higher risk of failure of methicillin-resistant Staphylococcus aureus prosthetic joint infections. Clin Orthop Relat Res. 2007. August;461:48-53. [DOI] [PubMed] [Google Scholar]
- 16.Walls RJ, Roche SJ, O’Rourke A, McCabe JP. Surgical site infection with methicillin-resistant Staphylococcus aureus after primary total hip replacement. J Bone Joint Surg Br. 2008. March;90(3):292-8. [DOI] [PubMed] [Google Scholar]
- 17.Howden BP, Davies JK, Johnson PD, Stinear TP, Grayson ML. Reduced vancomycin susceptibility in Staphylococcus aureus, including vancomycin-intermediate and heterogeneous vancomycin-intermediate strains: resistance mechanisms, laboratory detection, and clinical implications. Clin Microbiol Rev. 2010. January;23(1):99-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aslam S, Trautner BW, Ramanathan V, Darouiche RO. Combination of tigecycline and N-acetylcysteine reduces biofilm-embedded bacteria on vascular catheters. Antimicrob Agents Chemother. 2007. April;51(4):1556-8. Epub 2007 Jan 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cafiso V, Bertuccio T, Spina D, Purrello S, Stefani S. Tigecycline inhibition of a mature biofilm in clinical isolates of Staphylococcus aureus : comparison with other drugs. FEMS Immunol Med Microbiol. 2010. August;59(3):466-9. Epub 2010 May 19. [DOI] [PubMed] [Google Scholar]
- 20.Cai Y, Wang R, Liang B, Bai N, Liu Y. Systematic review and meta-analysis of the effectiveness and safety of tigecycline for treatment of infectious disease. Antimicrob Agents Chemother. 2011. March;55(3):1162-72. Epub 2010 Dec 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brand AM, de Kwaadsteniet M, Dicks LM. The ability of nisin F to control Staphylococcus aureus infection in the peritoneal cavity, as studied in mice. Lett Appl Microbiol. 2010. December;51(6):645-9. Epub 2010 Oct 11. [DOI] [PubMed] [Google Scholar]
- 22.Faust N, Varas F, Kelly LM, Heck S, Graf T. Insertion of enhanced green fluorescent protein into the lysozyme gene creates mice with green fluorescent granulocytes and macrophages. Blood. 2000. July 15;96(2):719-26. [PubMed] [Google Scholar]
- 23.Pribaz JR, Bernthal NM, Billi F, Cho JS, Ramos RI, Guo Y, Cheung AL, Francis KP, Miller LS. Mouse model of chronic post-arthroplasty infection: noninvasive in vivo bioluminescence imaging to monitor bacterial burden for long-term study. J Orthop Res. 2012. March;30(3):335-40. Epub 2011 Aug 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bernthal NM, Stavrakis AI, Billi F, Cho JS, Kremen TJ, Simon SI, Cheung AL, Finerman GA, Lieberman JR, Adams JS, Miller LS. A mouse model of post-arthroplasty Staphylococcus aureus joint infection to evaluate in vivo the efficacy of antimicrobial implant coatings. PLoS One. 2010. September 7;5(9):e12580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bernthal NM, Pribaz JR, Stavrakis AI, Billi F, Cho JS, Ramos RI, Francis KP, Iwakura Y, Miller LS. Protective role of IL-1β against post-arthroplasty Staphylococcus aureus infection. J Orthop Res. 2011. October;29(10):1621-6. Epub 2011 Mar 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bernthal NM, Taylor BN, Meganck JA, Wang Y, Shahbazian JH, Niska JA, Francis KP, Miller LS. Combined in vivo optical and μCT imaging to monitor infection, inflammation, and bone anatomy in an orthopaedic implant infection in mice. J Vis Exp. 2014. October 16;(92):e51612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cho JS, Pietras EM, Garcia NC, Ramos RI, Farzam DM, Monroe HR, Magorien JE, Blauvelt A, Kolls JK, Cheung AL, Cheng G, Modlin RL, Miller LS. IL-17 is essential for host defense against cutaneous Staphylococcus aureus infection in mice. J Clin Invest. 2010. May;120(5):1762-73. Epub 2010 Apr 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Darouiche RO. Treatment of infections associated with surgical implants. N Engl J Med. 2004. April 1;350(14):1422-9. [DOI] [PubMed] [Google Scholar]
- 29.Trampuz A, Widmer AF. Infections associated with orthopedic implants. Curr Opin Infect Dis. 2006. August;19(4):349-56. [DOI] [PubMed] [Google Scholar]
- 30.Miller LS, Pietras EM, Uricchio LH, Hirano K, Rao S, Lin H, O’Connell RM, Iwakura Y, Cheung AL, Cheng G, Modlin RL. Inflammasome-mediated production of IL-1beta is required for neutrophil recruitment against Staphylococcus aureus in vivo. J Immunol. 2007. November 15;179(10):6933-42. [DOI] [PubMed] [Google Scholar]
- 31.Antoci V Jr, King SB, Jose B, Parvizi J, Zeiger AR, Wickstrom E, Freeman TA, Composto RJ, Ducheyne P, Shapiro IM, Hickok NJ, Adams CS. Vancomycin covalently bonded to titanium alloy prevents bacterial colonization. J Orthop Res. 2007. July;25(7):858-66. [DOI] [PubMed] [Google Scholar]
- 32.Kurtz SM, Lau E, Schmier J, Ong KL, Zhao K, Parvizi J. Infection burden for hip and knee arthroplasty in the United States. J Arthroplasty. 2008. October;23(7):984-91. Epub 2008 Apr 10. [DOI] [PubMed] [Google Scholar]
- 33.Bozic KJ, Ries MD. The impact of infection after total hip arthroplasty on hospital and surgeon resource utilization. J Bone Joint Surg Am. 2005. August;87(8):1746-51. [DOI] [PubMed] [Google Scholar]
- 34.Stoodley P, Nistico L, Johnson S, Lasko LA, Baratz M, Gahlot V, Ehrlich GD, Kathju S. Direct demonstration of viable Staphylococcus aureus biofilms in an infected total joint arthroplasty. A case report. J Bone Joint Surg Am. 2008. August;90(8):1751-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sheehan E, McKenna J, Mulhall KJ, Marks P, McCormack D. Adhesion of Staphylococcus to orthopaedic metals, an in vivo study. J Orthop Res. 2004. January;22(1):39-43. [DOI] [PubMed] [Google Scholar]
- 36.Nishimura S, Tsurumoto T, Yonekura A, Adachi K, Shindo H. Antimicrobial susceptibility of Staphylococcus aureus and Staphylococcus epidermidis biofilms isolated from infected total hip arthroplasty cases. J Orthop Sci. 2006. January;11(1):46-50. [DOI] [PubMed] [Google Scholar]
- 37.van de Belt H, Neut D, Schenk W, van Horn JR, van Der Mei HC, Busscher HJ. Staphylococcus aureus biofilm formation on different gentamicin-loaded polymethylmethacrylate bone cements. Biomaterials. 2001. June;22(12):1607-11. [DOI] [PubMed] [Google Scholar]
- 38.Ji AJ, Saunders JP, Amorusi P, Wadgaonkar ND, O’Leary K, Leal M, Dukart G, Marshall B, Fluhler EN. A sensitive human bone assay for quantitation of tigecycline using LC/MS/MS. J Pharm Biomed Anal. 2008. November 4;48(3):866-75. Epub 2008 Jul 6. [DOI] [PubMed] [Google Scholar]
- 39.Landersdorfer CB, Bulitta JB, Kinzig M, Holzgrabe U, Sörgel F. Penetration of antibacterials into bone: pharmacokinetic, pharmacodynamic and bioanalytical considerations. Clin Pharmacokinet. 2009;48(2):89-124. [DOI] [PubMed] [Google Scholar]
- 40.Rodvold KA, Gotfried MH, Cwik M, Korth-Bradley JM, Dukart G, Ellis-Grosse EJ. Serum, tissue and body fluid concentrations of tigecycline after a single 100 mg dose. J Antimicrob Chemother. 2006. December;58(6):1221-9. Epub 2006 Sep 29. [DOI] [PubMed] [Google Scholar]
- 41.Lora-Tamayo J, Euba G, Ribera A, Murillo O, Pedrero S, García-Somoza D, Pujol M, Cabo X, Ariza J. Infected hip hemiarthroplasties and total hip arthroplasties: differential findings and prognosis. J Infect. 2013. December;67(6):536-44. Epub 2013 Aug 7. [DOI] [PubMed] [Google Scholar]
- 42.Maaloum Y, Meybeck A, Olive D, Boussekey N, Delannoy PY, Chiche A, Georges H, Beltrand E, Senneville E, d’Escrivan T, Leroy O. Clinical spectrum and outcome of critically ill patients suffering from prosthetic joint infections. Infection. 2013. April;41(2):493-501. Epub 2012 Oct 25. [DOI] [PubMed] [Google Scholar]
- 43.Widmer AF, Gaechter A, Ochsner PE, Zimmerli W. Antimicrobial treatment of orthopedic implant-related infections with rifampin combinations. Clin Infect Dis. 1992. June;14(6):1251-3. [DOI] [PubMed] [Google Scholar]
- 44.Zimmerli W, Widmer AF, Blatter M, Frei R, Ochsner PE; Foreign-Body Infection (FBI) Study Group. Role of rifampin for treatment of orthopedic implant-related staphylococcal infections: a randomized controlled trial. JAMA. 1998. May 20;279(19):1537-41. [DOI] [PubMed] [Google Scholar]
- 45.Prabhakara R, Harro JM, Leid JG, Keegan AD, Prior ML, Shirtliff ME. Suppression of the inflammatory immune response prevents the development of chronic biofilm infection due to methicillin-resistant Staphylococcus aureus. Infect Immun. 2011. December;79(12):5010-8. Epub 2011 Sep 26. [DOI] [PMC free article] [PubMed] [Google Scholar]