Abstract
Neutrophils have historically been characterized as first responder cells vital to host survival due to their ability to contain and eliminate bacterial and fungal pathogens. However, recent studies have shown that neutrophils participate in both protective and detrimental responses to a diverse array of inflammatory and infectious diseases. Although the contribution of neutrophils to extracellular infections has been investigated for decades, their specific role during intracellular bacterial infections has only recently been appreciated. During infection with the gram-positive intracellular pathogen Listeria monocytogenes, neutrophils are recruited from the bone marrow to sites of infection where they utilize novel bacterial sensing pathways leading to phagocytosis and production of bactericidal factors. This review summarizes the requirement of neutrophils during Listeria monocytogenes infection by examining both neutrophil trafficking and function during primary and secondary infection.
Introduction
Regulation of neutrophil development and death
Neutrophils are hematopoietic-derived immune cells that are generated, and continue to develop, in the bone marrow until recruited into circulation and then to sites of infection or inflammation. Steady-state neutrophil granulopoiesis is modulated by common stem cell cytokines, such as IL-3 and IL-6, as well as granulocyte colony stimulating factor (G-CSF) and granulocyte-macrophage colony stimulating factor (GM-CSF). Under infectious or inflammatory conditions, neutrophil granulopoiesis can be increased, typically termed “emergency granulopoiesis”, in order to restore homeostasis in the bone marrow after recruitment of neutrophils to peripheral sites (1). While IL-3, IL-6, G-CSF and GM-CSF have all been shown to contribute to emergency granulopoiesis, it has also been demonstrated that the production of reactive oxygen species (ROS) by bone marrow myeloid cells is critical for this process during infection (2). Neutrophils that traffic into tissues in the absence of infection or inflammation commonly become apoptotic rather than returning to circulation. It has been shown that these neutrophils are phagocytosed by resident macrophages and dendritic cells in the liver, which could potentially induce a feedback loop that decreases further granulopoiesis (3). Alternately, the chemokine receptor CXCR4 is upregulated as circulating neutrophils age, leading to trafficking back to the bone marrow where they are ingested by macrophages (4–6). Although neutrophil production is constitutive during homeostasis, an enhanced neutrophil response is often essential for host survival.
Neutrophils in disease
Neutrophils have a well-established role during fungal and extracellular bacterial infections where they promote bacterial clearance through phagocytosis, production of reactive oxygen and nitrogen species (ROS/RNS), neutrophil extracellular trap (NET) formation, and production of pro-inflammatory cytokines (6, 7). Recently, studies have focused on more non-traditional roles for neutrophils in disease including detrimental effects during inflammatory conditions, ranging from seasonal allergies to diabetes, protection against viral infections, and both protective and damaging effects during cancer (8). However, recent studies also suggest that neutrophils play an important protective role during some intracellular bacterial infections, including Listeria monocytogenes.
Listeria monocytogenes
Listeria monocytogenes is a gram-positive bacterium with a primarily intracellular life cycle after infection of a host organism. L. monocytogenes infection occurs following ingestion of contaminated foods and is the third leading cause of death among foodborne pathogens (9). Immunocompromised individuals, pregnant women and newborns are particularly susceptible to infection which can result in septicemia, meningitis, and loss of fetus. While foci of infection are generally established in the spleen and liver, L. monocytogenes can travel through the circulation to the heart, the brain, and to the bone marrow (10–12).
L. monocytogenes is able to cross the intestinal wall by binding to E-cadherin with one of its virulence factors, Internalin A. From the intestine, L. monocytogenes disseminates through the lymphatics and the bloodstream to the spleen and liver, where it can enter target cells via phagocytosis or induced endocytosis. In the liver, one of the primary target cells for infection are hepatocytes, which are initially directly infected through utilization of the virulence factor Internalin B binding to hepatocyte growth factor receptor (HGFR, Met, c-Met). Once internalized, L. monocytogenes is able to escape into the cytosol of host cells by secretion of listeriolysin O (LLO). Cytoplasmic L. monocytogenes then replicates and spreads to neighboring cells by polymerizing host actin with the aid of actin-assembly-inducing protein A (ActA) (13, 14). This indirect infection via cell-to-cell spread is effective, particularly in the liver, because it allows L. monocytogenes to infect neighboring cells without being exposed to opsonization or recognition and killing by innate immune cells.
Requirement of neutrophils during L. monocytogenes infection
Initial immune responses against L. monocytogenes are managed by innate immune cells, with macrophages, monocytes and neutrophils playing a central role. Early depletion studies using the anti-GR-1 monoclonal antibody (RB6-8C5) concluded that neutrophils are critically important for host defense during L. monocytogenes infection (15–21). More recently, it was shown that the anti-GR-1 antibody binds to Ly6G, which is expressed exclusively by neutrophils, and Ly6C, which is expressed by neutrophils, monocytes, subsets of dendritic cells, and subsets of memory CD8+ T cells (21, 22). However, use of the anti-Ly6G monoclonal antibody (1A8) for neutrophil-specific depletion studies has shown that neutrophils are essential for clearance of L. monocytogenes, particularly from the liver (23, 24). Our studies have also established that neutrophils are particularly important during high L. monocytogenes inoculum, consistent with previous reports indicating that following the administration of a high dose infection, neutrophils ingest L. monocytogenes in the liver (20, 24). Another recent report presented data that suggest neutrophils are not required for protection against L. monocytogenes. However, a relatively low dose of infection was used and importantly, neutrophils were depleted via intraperitoneal injection of the 1A8 antibody at the time of infection (25). It is possible that after intravenous infection and subsequent rapid arrival of L. monocytogenes at the target organs, neutrophils were still present and contributed to early bacterial uptake and killing. Further highlighting the importance of neutrophils, mice lacking G-CSF, or its receptor, display severe neutropenia and are more susceptible to L. monocytogenes infection than wild-type mice (26, 27). Additionally, an increased presence of neutrophils in peripheral organs, resulting in increased resistance against L. monocytogenes, was observed in mice deficient in B7-H4, a molecule which inhibits growth of neutrophil progenitors (28). Another study showed that over-activation of the innate immune response, by high-dose L. monocytogenes infection or pre-activation with either heat killed L. monocytogenes or synthetic TLR2-ligand followed by low-dose L. monocytogenes infection, led to significant neutrophil apoptosis in the bone marrow and a subsequent increase in host susceptibility (29).
Besides neutrophils, other phagocytic cells have well-established roles during L. monocytogenes infection. Depletion of splenic and liver macrophages during L. monocytogenes infection results in increased mortality and bacterial burden (30, 31). Tissue macrophages are also involved in the production of TNF-α and IL-12 in response to recognition of L. monocytogenes (32, 33). Inflammatory monocytes have been shown to respond to L. monocytogenes by producing IL-12 and IL-15 (34). Additional studies identified a differentiated population of CCR2+ inflammatory monocytes, also termed Tip-DCs, which produce both TNF-α and iNOS (inducible nitric oxide synthase) and are essential for clearance of L. monocytogenes infection (35). Ultimately, the production of innate cytokines from macrophages and inflammatory monocytes induces the production of IFN-γ from multiple cell types, leading to increased macrophage phagocytosis and killing of the bacteria (33, 36). The complex interplay between tissue resident and recruited phagocytic cells is critical for protection of the host against L. monocytogenes.
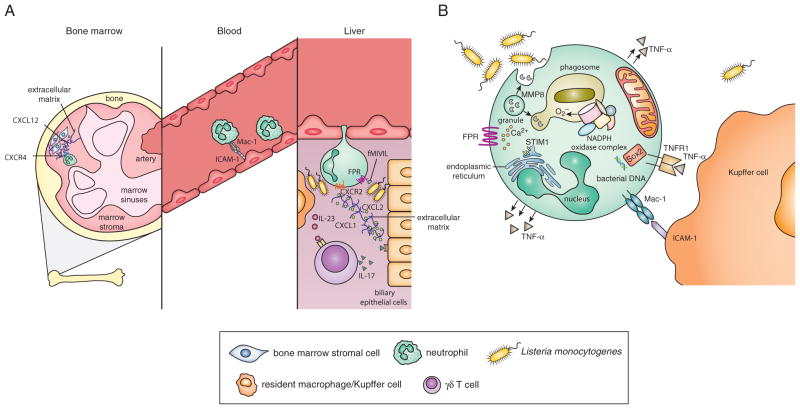
Neutrophil recruitment during L. monocytogenes infection
Neutrophil release from bone marrow
During homeostasis, neutrophils are retained in the bone marrow by the interaction of CXCR4 with its ligand CXCL12 (SDF-1), which is expressed by non-hematopoietic bone marrow cells. Following L. monocytogenes infection, neutrophils are rapidly recruited out of the bone marrow primarily due to the downregulation of CXCR4 followed by the upregulation of CXCR2, the receptor for neutrophil-attracting chemokines such as CXCL1 (KC) and CXCL2 (MIP-2α) (37, 38). Myeloid-lineage specific loss of CXCR4 was shown to lead to premature release of neutrophils from the bone marrow into the blood during basal conditions but also contributed to an impaired release of neutrophils in response to G-CSF, CXCL2 or L. monocytogenes infection (37). This suggests that during L. monocytogenes infection CXCR4 is essential for regulating neutrophil release from the bone marrow. Under basal conditions, G-CSF may also play an essential role in mobilization of neutrophils out of the bone marrow, likely by decreasing concentrations of CXCL12 (39).
Neutrophil recruitment from the bone marrow may also be induced during infection or inflammation by host-derived damage-associated molecular patterns (DAMPs) as well as pathogen-associated molecular patterns (PAMPs) (40, 41). There are no current studies illustrating L. monocytogenes-specific PAMPs that can induce neutrophil release or the role of CXCL1, CXCL2 or CXCL12 in the bone marrow during infection. However, one could speculate that increased concentrations of G-CSF induced by L. monocytogenes infection would lead to decreased concentrations of CXCL12 followed by a downregulation in neutrophil CXCR4 expression and enhanced release of neutrophils from the bone marrow into circulation.
Neutrophil extravasation into tissue
During L. monocytogenes infection, neutrophils that are released from the bone marrow are subsequently recruited to infected organs, primarily the liver. Efficient neutrophil chemotaxis is dependent on chemoattractant molecules that induce signaling pathways leading to the rearrangement of intracellular structural molecules and upregulation of surface adhesion molecules. Formylated peptide receptors (FPRs) are highly expressed on neutrophils and can bind L. monocytogenes-derived formylated peptides (such as fMIVIL) resulting in a signaling cascade that induces neutrophil migration (42). Accordingly, mice deficient in FPR1, FPR2 or both, are more susceptible to L. monocytogenes infection and have delayed recruitment of neutrophils to the liver (42–44). This occurs in the absence of differences in concentrations of common neutrophil-attracting chemokines, suggesting that FPRs are responsible for initial chemotactic signals to recruit neutrophils into the liver during L. monocytogenes infection (43, 44).
The neutrophil-attracting chemokines CXCL1 and CXCL2 are produced in the liver following L. monocytogenes infection (38). Treatment with anti-CXCL2 (anti-MIP-2) antibody decreases neutrophil recruitment to the liver after L. monocytogenes infection in wild-type mice and antibody blockade of CXCR2 completely ablated efficient neutrophil recruitment (38). Conversely, mice deficient in the murine IL-8 receptor homolog (CXCR2) have previously been shown to be more resistant to acute L. monocytogenes infection (45). However, this increased resistance to infection is likely attributed to the extreme neutrophilia observed in these mice (46, 47). Mice lacking the type I interferon receptor (IFNAR) show increased neutrophil recruitment to sites of infection and increased resistance to L. monocytogenes infection compared to wild-type mice. Pharmacological inhibition of CXCR2 in IFNAR deficient mice reversed both the enhanced neutrophil recruitment and the increased resistance to infection] (48). Collectively, these data suggest that FPRs are required for initial extravasation into the liver with subsequent chemokine receptor signaling implicated in neutrophil recruitment to the site of L. monocytogenes infection within the tissue.
Mature neutrophils upregulate expression of adhesion molecules resulting in efficient extravasation into tissues. Specific adhesion molecules expressed on neutrophils include LFA-1 (CD11a/CD18) and Mac-1 (CD11b/CD18, CR3) (49). Interestingly, CD18 KO mice (deficient for LFA-1 and Mac-1) display increased resistance to L. monocytogenes infection, probably due to increased presence of neutrophils in the periphery caused by increased concentrations of G-CSF (50). Similarly, mice deficient in CD11a (LFA-1) are more resistant to L. monocytogenes infection, have increased infiltration of neutrophils into the liver, and increased concentrations of both G-CSF and IL-17. Furthermore, neutrophil depletion (using the GR-1 antibody) in LFA-1 deficient mice abrogated the increased resistance to L. monocytogenes (51). Conversely, antibody blockade of CD11b (Mac-1) results in a reduction of neutrophil recruitment to the site of L. monocytogenes infection in the liver and decreased resistance to L. monocytogenes infection (30, 52). Collectively, these data suggest that LFA-1 expression restricts neutrophil recruitment to sites of infection leading to increased bacterial burden, while Mac-1, particularly the CD11b component, is essential for neutrophil recruitment and control of L. monocytogenes infection.
Indirect regulation of neutrophil chemotaxis
During L. monocytogenes infection, IL-23 regulates the production of IL-17A and IL-17F from γδ T cells, resulting in optimal liver neutrophil recruitment and enhanced bacterial clearance presumably due to increased chemokine production. Mice lacking IL-23p19, IL-17A, or IL-17RA have increased bacterial burdens in the liver, which corresponds with decreased neutrophil recruitment (53, 54). One study showed that increased concentrations of CCL8 (MCP-2) led to an influx of IL-17-producing γδ T cells in mice conditionally knocked out for B lymphocyte-induced maturation protein-1 (BLIMP1) in macrophages. This resulted in a subsequent enhancement of neutrophil recruitment to sites of infection and increased clearance of L. monocytogenes (55). These studies demonstrate the importance of chemokine regulation in effective neutrophil recruitment and ultimately, clearance of bacteria.
Other cytokines that have been suggested to be important during L. monocytogenes infection, likely through indirect effects on neutrophils, include IL-1 and IL-6. IL-1α and IL-1β are produced in the liver and spleen after L. monocytogenes infection and exogenous IL-1α has been shown to increase neutrophil recruitment to sites of infection and decrease bacterial burden (56–59). Blocking the type 1 IL-1 receptor (IL-1R), which binds both IL-1α and IL-1β, leads to increased susceptibility to L. monocytogenes (60). Interestingly, IL-1β deficient mice show no difference in susceptibility to L. monocytogenes, suggesting a more central role for IL-1α (61). Studies performing IL-1 depletion in SCID mice show increased susceptibility to L. monocytogenes compared to untreated SCID mice, suggesting the mechanism of IL-1 protection is not mediated by T cells (62). However, further studies are required to determine how IL-1α mediates its protective effects, particularly in relation to neutrophils, during L. monocytogenes infection.
Mice deficient for IL-6 are more susceptible to L. monocytogenes infection with increased bacterial burden in both the spleen and liver and deficient neutrophil recruitment into the blood compared to wild-type mice (63, 64). Addition of rIL-6 to wild-type mice was able to provide enhanced protection against L. monocytogenes. Use of the anti-GR-1 antibody for depletion, though not specific for neutrophils, eliminated the IL-6 induced protective effect, suggesting IL-6 can directly or indirectly enhance recruitment, and possibly function, of neutrophils (63). Interestingly, in IL-6 KO mice no differences were observed in NK cell or macrophage activation and there was no difference in IFN-γ production during L. monocytogenes infection, further highlighting the potential link between IL-6 and neutrophils (63). Although it has been shown that classical IL-6 signaling, rather than IL-6 trans-signaling, is required for protection against L. monocytogenes, the cells responsible for producing and responding to IL-6 during infection are not known (65).
Extracellular superoxide dismutase (ecSOD) is the enzyme responsible for regulating extracellular concentrations of ROS and protecting host tissues during inflammation. Our lab has shown that ecSOD activity results in increased neutrophil recruitment to the liver during L. monocytogenes infection, possibly facilitated by the enzyme’s ability to protect the extracellular matrix from degradation leading to enhanced neutrophil trafficking. However, the increased number of neutrophils did not correlate with protection during infection, as mice with high ecSOD activity are more susceptible to L. monocytogenes than mice with wild-type ecSOD activity or mice deficient in ecSOD. Furthermore, in mice with high ecSOD activity, neutrophils did not effectively co-localize with bacterial lesions in the liver, suggesting not only chemotactic, but potentially functional defects (66). Ultimately, rapid recruitment out of the bone marrow and efficient chemotaxis to sites of infection are essential preludes to neutrophil function and clearance of L. monocytogenes infection.
Neutrophil function during L. monocytogenes infection
Neutrophil phagocytosis and containment of bacteria
Upon recruitment to foci of infection, particularly within the liver, neutrophils recognize and phagocytose L. monocytogenes. Specific receptors, including the complement receptor of the immunoglobulin superfamily (CRIg), have been shown to be required for macrophage phagocytosis of L. monocytogenes (67); however, the receptors and ligands that induce phagocytosis by neutrophils are currently unknown. The majority of bacteria recovered from the liver immediately following high-dose intravenous infection with L. monocytogenes are presumed to be extracellular and many are associated with hepatocytes. However, the rapid influx of neutrophils to the liver during the first 6 hours post-infection leads to a significant reduction in the bacterial burden. In addition, depletion using the anti-GR-1 antibody prior to infection, though not specific for neutrophils, led to markedly increased hepatocyte damage and increased bacterial burden (20). Collectively, these data suggest that early phagocytosis of L. monocytogenes by incoming neutrophils is essential for protection of the liver against infection.
Neutrophil phagocytosis of bacteria may not necessarily result in bacterial killing but may instead limit the spread of L. monocytogenes. The bacteria-filled neutrophils could then be phagocytosed by macrophages, ultimately killing the bacteria. One study demonstrated the presence of L. monocytogenes inside liver neutrophils that were located inside Kupffer cells (30). Furthermore, the Mac-1 receptor on neutrophils can bind to ICAM-1 (CD54) on the surface of macrophages and potentially facilitate phagocytosis of infected neutrophils by Kupffer cells. It has been shown that inhibition of either CD11b (Mac-1) or ICAM-1 resulted in a reduced clearance of L. monocytogenes in the liver, although this could be related to altered recruitment in addition to blockade of neutrophil-macrophage interactions (30). Therefore, one could speculate that the contribution of neutrophils to protection against L. monocytogenes infection is purely related to bacterial containment.
Neutrophils produce matrix metalloproteinase-8 (MMP8) and store it in granules until bacterial sensing induces degranulation at sites of phagocytosis allowing MMP8 to be taken into the phagosome with L. monocytogenes where it was shown to degrade LLO. It was further proposed that this leads to bacterial containment by preventing L. monocytogenes escape from the phagosome. These in vitro studies showed that inhibition of neutrophil degranulation led to increased cell damage and inhibition of proteases led to decreased LLO degradation and increased intracellular neutrophil bacterial burden, supporting a potential role for MMP8 and other granule contents in protecting the host against L. monocytogenes infection (68).
Neutrophil production of ROS
Direct sensing of L. monocytogenes by murine neutrophils is thought to be mediated primarily by formylated peptides binding to the receptor FPR1 on neutrophils, which induces a signaling cascade leading to calcium efflux and subsequent superoxide (O2·−) and hydrogen peroxide (H2O2) production (42). Lack of FPR1 results in increased bacterial burden and decreased production of O2·− and H2O2 by neutrophils (43). Regulation of calcium entry is an important factor in neutrophil function, including production of ROS, and is mediated by molecules such as stromal-interacting molecule 1 (STIM1). Mice deficient in STIM1 have decreased production of ROS and increased susceptibility to L. monocytogenes infection (69). Mice deficient for 4-1BB (CD137), a member of the TNF receptor superfamily constitutively expressed by neutrophils, are more susceptible to L. monocytogenes infection than wild-type mice, which correlates with defective calcium mobilization and decreased ROS production from neutrophils (70). Furthermore, pre-treatment of wild-type mice with a 4-1BB agonist antibody led to decreased bacteria burden and increased neutrophil ROS production suggesting a role for TNF receptors in activation of signaling pathways leading to production of ROS during L. monocytogenes infection (71).
Neutrophil activation during L. monocytogenes infection induces production of O2·− and H2O2, both of which are anti-microbial and thought to be important for bacterial killing. The NADPH oxidase complex assembles on the phagosome in neutrophils and converts molecular oxygen (O2) into O2·− (72). The NADPH oxidase complex is comprised of 6 subunits: gp91phox and p22phox are membrane-bound components while p47phox, p67phox, and p40phox are all cytosolic components that assemble with the membrane-bound portion, and either Rac1 or Rac2 GTPases, upon activation of the cell (73). Mice that lack the essential gp91phox component of NADPH oxidase are more susceptible to L. monocytogenes during the early stages of infection (74–76). Interestingly, mice deficient in the p47phox component have equivalent bacterial burden to wild-type mice during L. monocytogenes infection (77). Lack of the p47phox subunit may be compensated for by high concentrations of the p67phox subunit, leading to efficient O2·− production; however, this has not been thoroughly investigated in vivo (78). In addition to ROS production by the NADPH oxidase complex, it has been shown that mitochondria-generated ROS is important for phagocyte-mediated bacterial killing, although L. monocytogenes was not used in this study (79). Furthermore, efficient phagosome localization with the mitochondria, mediated by the Mst1 and Mst2 kinases, is required for optimal induction of ROS downstream of TLR signaling, and mice lacking both Mst1 and Mst2 show increased susceptibility to L. monocytogenes compared to wild-type mice (80).
While ROS are potent bactericidal molecules, they can also cause host tissue damage and must therefore be properly regulated. The negative regulator of ROS (NRROS) is a recently described protein important for preventing tissue damage to host organs by limiting phagocytic production of ROS. Increased ROS production, increased resistance to L. monocytogenes infection, and increased tissue damage, were all observed in mice deficient in NRROS (81). The O2·− generated by NADPH oxidase is converted into H2O2 by superoxide dismutases (SODs). In the extracellular milieu, ecSOD catalyzes the conversion of O2·− to H2O2 to protect the host from excessive tissue damage. Our lab has previously shown that during L. monocytogenes infection, mice with high ecSOD activity have increased bacterial burden and neutrophil apoptosis as well as impaired neutrophil-specific production of TNF-α, compared to ecSOD wild-type or ecSOD KO mice. Depletion of neutrophils in mice with high ecSOD activity slightly decreases bacterial burden while neutrophil depletion in ecSOD wild-type or ecSOD KO mice results in increased bacterial burden, suggesting ecSOD activity leads to impaired neutrophil function (66). Though O2·− and H2O2 are produced by activated neutrophils, hypochlorous acid (HOCl) is believed to be a more potent bactericidal ROS molecule (82, 83). Production of HOCl is catalyzed by myeloperoxidase (MPO) in the presence of H2O2 and chloride ions. Neutrophil-specific MPO activity against L. monocytogenes has not been determined, though it has been implicated as being important for neutrophil anti-microbial activity against other bacterial pathogens (84).
In addition to ROS, neutrophils can generate reactive nitrogen species (RNS) through the expression of inducible nitric oxide synthase (iNOS or NOS2), an enzyme that converts O2 to nitric oxide (NO·). NOS2 deficient mice were found to be more susceptible to L. monocytogenes infection than wild-type mice (75, 85). Conversely, a more recent study showed that pharmacologic inhibition of NOS2 resulted in decreased bacteria burden in the liver after infection with L. monocytogenes (86). Why different approaches to eliminating NOS2 function resulted in different outcomes, and importantly, whether or not NOS2 is required for neutrophil killing in vivo during L. monocytogenes infection still needs to be resolved. Peroxynitrite (NO3·−), a ROS molecule produced by a reaction between O2·− and NO·, is also thought to have very potent bactericidal activities, including the ability to kill L. monocytogenes in vitro (87). However, these activities have not yet been identified as neutrophil specific, nor have they been shown to be required for killing in vivo.
Neutrophil production of cytokines
Neutrophil activation induced by L. monocytogenes infection also results in the production of several cytokines that have been deemed important for resistance to bacterial infection. Generally, recognition of microbial products by pattern recognition receptors (PRRs) initiates signaling pathways through adaptor molecules leading to activation of the NF-κB transcription factor and ultimately, production of cytokines.
It has been shown that mice lacking MyD88 (a TLR adaptor protein) are very susceptible to L. monocytogenes infection, have reduced production of IL-6, IL-12, IL-18, IFN-γ and TNF-α and decreased neutrophil recruitment to the spleen (32, 88). Mice with MyD88 expression exclusive to dendritic cells responded comparably to wild-type mice during infection with L. monocytogenes (89). These data suggest that MyD88 is required in dendritic cells for optimal responses to L. monocytogenes but neutrophil recruitment and function are independent of MyD88 signaling.
While TLRs are responsible for recognizing a wide array of extracellular or vesicular pathogens, Nod-like receptors (NLRs) are positioned in the cytosol in order to recognize PAMPs expressed by pathogens that escape the phagosome. Mice deficient in NOD1 have increased bacterial burden during L. monocytogenes infection which correlates with a decrease in neutrophil recruitment. However, it was determined that NOD1 signaling was essential in non-hematopoietic, but not hematopoietic cells, during infection (90). Furthermore, neutrophils from RIP2 KO mice, which cannot signal through NOD1 or NOD2, did not display altered production of IL-6, TNF-α, CXCL1 or CXCL2 in response to L. monocytogenes infection, as compared to neutrophils from wild-type mice (91). This suggests that NLR signaling may be a redundant, rather than essential, pathway to induce neutrophil cytokine production during infection with L. monocytogenes. One member of the NLR family, NLRP6, has been suggested to be a negative regulator of inflammatory responses during infection with L. monocytogenes. Mice deficient in NLRP6 show increased survival and decreased bacterial burden following L. monocytogenes infection. In addition, NLRP6-deficient mice exhibit increased IL-6 and CXCL1 concentrations in circulation and in the peritoneum correlating with increased recruitment of GR-1+ cells to sites of infection (92).
A recent study identified the Sox2 transcription factor acting in the cytosol of neutrophils as a sensor of bacterial DNA. Upon recognition of bacterial DNA, such as that from L. monocytogenes, Sox2 initiates a signaling cascade ultimately resulting in production of pro- inflammatory cytokines, including TNF-α and IL-6. In addition, mice with phagocyte-specific Sox2 deficiency exhibited increased susceptibility to L. monocytogenes infection and since Sox2 is not expressed in macrophages, this indicates that Sox2 is a novel and essential sensor of L. monocytogenes in neutrophils (93). Mice deficient in Toso, an Fc receptor for IgM with previously unknown function, predominantly displayed decreased production of TNF-α, IL-6, and IL-12, as well as decreased phagocytic ability by granulocytes and a concurrent increase in bacterial burden following L. monocytogenes infection (94). These recent studies highlight novel bacterial sensors and signaling pathways in neutrophil activation during L. monocytogenes infection.
Deficiency in IFN-γ or the IFN-γ receptor renders mice highly susceptible to infection with L. monocytogenes (32, 36, 95, 96). Multiple subsets of lymphocytes can produce IFN-γ during L. monocytogenes infection (97), and our studies have shown that antigen-independent responses of memory CD8+ T cells are superior to NK cells at providing protection when transferred into IFN-γ deficient hosts (98). Interestingly, one study observed neutrophil-specific production of IFN-γ during L. monocytogenes infection and showed that transferring neutrophils from wild-type mice into IFN-γ KO mice increased bacterial clearance (99), suggesting that multiple cells have the capacity to provide IFN-γ mediated protection against L. monocytogenes. Mice deficient in TNF-α, a pro-inflammatory cytokine produced by immune cells, or its receptor TNFR1, are highly susceptible to L. monocytogenes infection (100–103). Additionally, mice conditionally knocked out for phagocyte-specific TNF-α display extreme susceptibility to L. monocytogenes infection characterized by increased bacterial burden in the spleen and liver and decreased host survival (104). Furthermore, depletion of neutrophils during L. monocytogenes infection decreases the amount of TNF-α produced, correlating with increased bacterial burden (24). Decreased neutrophil activation due to deficiency of FPR1 or 4-1BB, also resulted in a decrease in TNF-α production (42, 71). Although the precise role of TNF-α produced by neutrophils during L. monocytogenes infection is not known, it is possible that hepatocytes are lysed by the actions of neutrophil specific TNF-α production. A previous study has shown that neutrophil depletion with the GR-1 antibody led to increased liver damage assessed by increased AST concentrations in serum (30). Additional studies using microscopy have suggested that depletion of neutrophils with the GR-1 antibody results in decreased hepatocyte death at 24 hours post-infection (16). Furthermore, TNF-α has been shown to directly induce hepatocyte lysis (105, 106). Initially, neutrophil phagocytosis of L. monocytogenes could limit hepatocyte infection and at later time points neutrophil TNF-α production could induce hepatocyte death, thus reducing the cell-to-cell spread of L. monocytogenes.
Importance of neutrophils during secondary L. monocytogenes infection
Initial depletion studies using the anti-GR-1 antibody (RB6-8C5) concluded that neutrophils are critically important for host defense during secondary L. monocytogenes infection (18, 107). However, since it is known that anti-GR-1 depletes multiple cell types in addition to neutrophils, interpretation of these data is challenging. Recent studies in our lab using the anti-Ly6G antibody (1A8) have shown that depletion of neutrophils during a secondary L. monocytogenes infection results in increased bacterial burden in both the spleen and liver (unpublished data). Additional studies have shown that memory CD8+ T cells rapidly produce CCL3 and IFN-γ during secondary L. monocytogenes infection (108–110). Interestingly, CCL3 induces recruitment of TNF-α producing monocytes resulting in ROS production from both monocytes and neutrophils (108, 109). The transfer of neutrophils into wild-type mice during secondary infection enhanced bacterial clearance, but only if the neutrophils expressed p47phox, suggesting ROS production is required for neutrophil protection during a secondary response to L. monocytogenes (95). Although this finding contrasts with the expendable role of p47phox during a primary infection, the disparate results are likely due to differences in NADPH oxidase component requirements and the potential compensation via p67phox in primary compared to secondary immune responses to L. monocytogenes. Collectively, these studies highlight an important role for neutrophils, and other innate immune cells, in clearance of secondary L. monocytogenes infection.
Conclusions
Despite the fact that neutrophils have long been considered an integral part of the innate immune response, the role of these granulocytic cells in disease and inflammation continues to expand. Although recent studies highlight the importance of neutrophil recruitment and function during both primary and secondary infection with L. monocytogenes, many questions still remain unanswered (Table 1, Figure 1). Existing studies have focused almost exclusively on the role of neutrophils in the liver and spleen during L. monocytogenes infection; however, potential involvement of neutrophils during infection in the bone marrow, intestines, and even the central nervous system, remains unclear. A recent report implicated neutrophils in recruiting antigen specific CD8+ T cells during pregnancy, resulting in spontaneous resorptions, thus indicating that although neutrophils are protective during L. monocytogenes infection in the liver, they are detrimental during pregnancy (111). Neutrophil recruitment and function during other intracellular bacterial, protozoan, and viral infections is a largely unexplored area of research. With the use of specific tools, including the 1A8 antibody to specifically deplete neutrophils, Genista mice lacking neutrophils (112), or conditional knockout mice expressing neutrophil-specific Cre, our mechanistic understanding of neutrophils should continue to expand. Armed with this knowledge, therapeutic interventions aimed at enhancing or blocking neutrophil recruitment or function can be applied to human diseases.
Table 1.
Molecules implicated in neutrophil recruitment and function during L. monocytogenes infection
| Factor | Effect on neutrophil recruitment | Effect on neutrophil function | Effect on resistance to L. monocytogenes | References |
|---|---|---|---|---|
| G-CSF (KO) | General neutropenia | Unknown | Decreased | (25, 26) |
| G-CSFR (KO) | General neutropenia | Unknown | Decreased | (25, 26) |
| B7-H4 (KO) | Increased recruitment to sites of infection | No effect on ROS or phagocytosis | Increased | (27) |
| FPR1 and FPR2 (KO) | Delayed recruitment to liver | Decreased TNF-α and ROS production | Decreased | (41–43) |
| IL-23p19 (KO) | Deficient recruitment to liver | Unknown | Decreased | (53) |
| IL-17RA (KO) | Deficient recruitment to liver | Unknown | Decreased | (53) |
| IL-17A (KO) | Deficient recruitment to liver | Unknown | Decreased | (52) |
| BLIMP1 (KO) | Increased recruitment | Unknown | Increased | (54) |
| IL-6 (KO) | Decreased recruitment into circulation | Unknown | Decreased | (64, 65) |
| ecSOD (KO) | Decreased recruitment to liver | Enhanced TNF-α production | Increased | (67) |
| CD11a (KO) | Increased recruitment to liver | Unknown | Increased | (50) |
| CD11b (blocking) | Reduced recruitment to liver | Unknown | Decreased | (29, 51) |
| CD18 (KO) | General neutrophilia | Unknown | Increased | (49) |
| STIM1 (KO) | No effect | Decreased ROS production | Decreased | (70) |
| gp91phox/NADPH oxidase (KO) | Unknown | Unknown | Decreased | (75–77) |
| P47phox (KO) | Unknown | Unknown | No effect | (78) |
| NRROS (KO) | Unknown | Unknown | Increased | (82) |
| iNOS/NOS2 (KO) (blocking) | Unknown | Unknown | KO: Decreased Blocking: Increased in liver; no effect in spleen | KO:(76, 86) Blocking: (87) |
| Sox2 (phagocyte- specific KO; not expressed in macrophages) | No effect on recruitment into blood; unknown effect on recruitment to sites of infection | Defective bacterial sensing | Decreased | (94) |
| Toso (KO) | Unknown | Decreased phagocytosis and impaired cytokine production | Decreased | (95) |
| 4-1BB/CD137 (KO) | Unknown | Decreased TNF-α and ROS production | Decreased | (71) |
| TNF-α (Phagocyte-specific KO) | Unknown | Defective TNF-α production | Decreased | (100) |
Figure 1.
Acknowledgments
Funding sources: NIH-AI109630 (REB), UNTHSC seed grant (REB) and AAI Careers in Immunology Fellowship (ARW)
References
- 1.Manz MG, Boettcher S. Emergency granulopoiesis. Nat Rev Immunol. 2014;14:302–314. doi: 10.1038/nri3660. [DOI] [PubMed] [Google Scholar]
- 2.Kwak HJ, Liu P, Bajrami B, Xu Y, Park SY, Nombela-Arrieta C, Mondal S, Sun Y, Zhu H, Chai L, Silberstein LE, Cheng T, Luo HR. Myeloid cell-derived reactive oxygen species externally regulate the proliferation of myeloid progenitors in emergency granulopoiesis. Immunity. 2015;42:159–171. doi: 10.1016/j.immuni.2014.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stark MA, Huo Y, Burcin TL, Morris MA, Olson TS, Ley K. Phagocytosis of apoptotic neutrophils regulates granulopoiesis via IL-23 and IL-17. Immunity. 2005;22:285–294. doi: 10.1016/j.immuni.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 4.Strydom N, Rankin SM. Regulation of circulating neutrophil numbers under homeostasis and in disease. J Innate Immun. 2013;5:304–314. doi: 10.1159/000350282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Casanova-Acebes M, Pitaval C, Weiss LA, Nombela-Arrieta C, Chevre R, AGN, Kunisaki Y, Zhang D, van Rooijen N, Silberstein LE, Weber C, Nagasawa T, Frenette PS, Castrillo A, Hidalgo A. Rhythmic modulation of the hematopoietic niche through neutrophil clearance. Cell. 2013;153:1025–1035. doi: 10.1016/j.cell.2013.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nauseef WM, Borregaard N. Neutrophils at work. Nat Immunol. 2014;15:602–611. doi: 10.1038/ni.2921. [DOI] [PubMed] [Google Scholar]
- 7.Mocsai A. Diverse novel functions of neutrophils in immunity, inflammation, and beyond. J Exp Med. 2013;210:1283–1299. doi: 10.1084/jem.20122220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mayadas TN, Cullere X, Lowell CA. The multifaceted functions of neutrophils. Annu Rev Pathol. 2014;9:181–218. doi: 10.1146/annurev-pathol-020712-164023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scallan E, Hoekstra RM, Angulo FJ, Tauxe RV, Widdowson MA, Roy SL, Jones JL, Griffin PM. Foodborne illness acquired in the United States--major pathogens. Emerg Infect Dis. 2011;17:7–15. doi: 10.3201/eid1701.P11101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hardy J, Chu P, Contag CH. Foci of Listeria monocytogenes persist in the bone marrow. Dis Model Mech. 2009;2:39–46. doi: 10.1242/dmm.000836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Join-Lambert OF, Ezine S, Le Monnier A, Jaubert F, Okabe M, Berche P, Kayal S. Listeria monocytogenes-infected bone marrow myeloid cells promote bacterial invasion of the central nervous system. Cell Microbiol. 2005;7:167–180. doi: 10.1111/j.1462-5822.2004.00444.x. [DOI] [PubMed] [Google Scholar]
- 12.Alonzo F, 3rd, Bobo LD, Skiest DJ, Freitag NE. Evidence for subpopulations of Listeria monocytogenes with enhanced invasion of cardiac cells. J Med Microbiol. 2011;60:423–434. doi: 10.1099/jmm.0.027185-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pamer EG. Immune responses to Listeria monocytogenes. Nat Rev Immunol. 2004;4:812–823. doi: 10.1038/nri1461. [DOI] [PubMed] [Google Scholar]
- 14.Cossart P, Lecuit M. Interactions of Listeria monocytogenes with mammalian cells during entry and actin-based movement: bacterial factors, cellular ligands and signaling. EMBO J. 1998;17:3797–3806. doi: 10.1093/emboj/17.14.3797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Czuprynski CJ, Brown JF, Maroushek N, Wagner RD, Steinberg H. Administration of anti-granulocyte mAb RB6-8C5 impairs the resistance of mice to Listeria monocytogenes infection. J Immunol. 1994;152:1836–1846. [PubMed] [Google Scholar]
- 16.Conlan JW, North RJ. Neutrophils are essential for early anti-Listeria defense in the liver, but not in the spleen or peritoneal cavity, as revealed by a granulocyte-depleting monoclonal antibody. J Exp Med. 1994;179:259–268. doi: 10.1084/jem.179.1.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Conlan JW. Critical roles of neutrophils in host defense against experimental systemic infections of mice by Listeria monocytogenes, Salmonella typhimurium, and Yersinia enterocolitica. Infect Immun. 1997;65:630–635. doi: 10.1128/iai.65.2.630-635.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rakhmilevich AL. Neutrophils are essential for resolution of primary and secondary infection with Listeria monocytogenes. J Leukoc Biol. 1995;57:827–831. doi: 10.1002/jlb.57.6.827. [DOI] [PubMed] [Google Scholar]
- 19.Rogers HW, Unanue ER. Neutrophils are involved in acute, nonspecific resistance to Listeria monocytogenes in mice. Infect Immun. 1993;61:5090–5096. doi: 10.1128/iai.61.12.5090-5096.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gregory SH, Sagnimeni AJ, Wing EJ. Bacteria in the bloodstream are trapped in the liver and killed by immigrating neutrophils. J Immunol. 1996;157:2514–2520. [PubMed] [Google Scholar]
- 21.Fleming TJ, Fleming ML, Malek TR. Selective expression of Ly-6G on myeloid lineage cells in mouse bone marrow. RB6-8C5 mAb to granulocyte-differentiation antigen (Gr-1) detects members of the Ly-6 family. J Immunol. 1993;151:2399–2408. [PubMed] [Google Scholar]
- 22.Daley JM, Thomay AA, Connolly MD, Reichner JS, Albina JE. Use of Ly6G-specific monoclonal antibody to deplete neutrophils in mice. J Leukoc Biol. 2008;83:64–70. doi: 10.1189/jlb.0407247. [DOI] [PubMed] [Google Scholar]
- 23.Edelson BT, Bradstreet TR, Hildner K, Carrero JA, Frederick KE, Kc W, Belizaire R, Aoshi T, Schreiber RD, Miller MJ, Murphy TL, Unanue ER, Murphy KM. CD8alpha(+) dendritic cells are an obligate cellular entry point for productive infection by Listeria monocytogenes. Immunity. 2011;35:236–248. doi: 10.1016/j.immuni.2011.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carr KD, Sieve AN, Indramohan M, Break TJ, Lee S, Berg RE. Specific depletion reveals a novel role for neutrophil-mediated protection in the liver during Listeria monocytogenes infection. Eur J Immunol. 2011;41:2666–2676. doi: 10.1002/eji.201041363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shi C, Hohl TM, Leiner I, Equinda MJ, Fan X, Pamer EG. Ly6G+ neutrophils are dispensable for defense against systemic Listeria monocytogenes infection. J Immunol. 2011;187:5293–5298. doi: 10.4049/jimmunol.1101721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lieschke GJ, Grail D, Hodgson G, Metcalf D, Stanley E, Cheers C, Fowler KJ, Basu S, Zhan YF, Dunn AR. Mice lacking granulocyte colony-stimulating factor have chronic neutropenia, granulocyte and macrophage progenitor cell deficiency, and impaired neutrophil mobilization. Blood. 1994;84:1737–1746. [PubMed] [Google Scholar]
- 27.Zhan Y, Lieschke GJ, Grail D, Dunn AR, Cheers C. Essential roles for granulocyte-macrophage colony-stimulating factor (GM-CSF) and G-CSF in the sustained hematopoietic response of Listeria monocytogenes-infected mice. Blood. 1998;91:863–869. [PubMed] [Google Scholar]
- 28.Zhu G, Augustine MM, Azuma T, Luo L, Yao S, Anand S, Rietz AC, Huang J, Xu H, Flies AS, Flies SJ, Tamada K, Colonna M, van Deursen JM, Chen L. B7-H4-deficient mice display augmented neutrophil-mediated innate immunity. Blood. 2009;113:1759–1767. doi: 10.1182/blood-2008-01-133223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Navarini AA, Lang KS, Verschoor A, Recher M, Zinkernagel AS, Nizet V, Odermatt B, Hengartner H, Zinkernagel RM. Innate immune-induced depletion of bone marrow neutrophils aggravates systemic bacterial infections. Proc Natl Acad Sci USA. 2009;106:7107–7112. doi: 10.1073/pnas.0901162106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gregory SH, Cousens LP, van Rooijen N, Dopp EA, Carlos TM, Wing EJ. Complementary Adhesion Molecules Promote Neutrophil- Kupffer Cell Interaction and the Elimination of Bacteria Taken Up by the Liver. J Immunol. 2002;168:308–315. doi: 10.4049/jimmunol.168.1.308. [DOI] [PubMed] [Google Scholar]
- 31.Pinto AJ, Stewart D, van Rooijen N, Morahan PS. Selective depletion of liver and splenic macrophages using liposomes encapsulating the drug dichloromethylene diphosphonate: effects on antimicrobial resistance. J Leukoc Biol. 1991;49:579–586. doi: 10.1002/jlb.49.6.579. [DOI] [PubMed] [Google Scholar]
- 32.Seki E, Tsutsui H, Tsuji NM, Hayashi N, Adachi K, Nakano H, Futatsugi-Yumikura S, Takeuchi O, Hoshino K, Akira S, Fujimoto J, Nakanishi K. Critical Roles of Myeloid Differentiation Factor 88-Dependent Proinflammatory Cytokine Release in Early Phase Clearance of Listeria monocytogenes in Mice. J Immunol. 2002;169:3863–3868. doi: 10.4049/jimmunol.169.7.3863. [DOI] [PubMed] [Google Scholar]
- 33.Tripp CS, Wolf SF, Unanue ER. Interleukin 12 and tumor necrosis factor alpha are costimulators of interferon gamma production by natural killer cells in severe combined immunodeficiency mice with listeriosis, and interleukin 10 is a physiologic antagonist. Proc Natl Acad Sci USA. 1993;90:3725–3729. doi: 10.1073/pnas.90.8.3725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Soudja SM, Ruiz AL, Marie JC, Lauvau G. Inflammatory monocytes activate memory CD8(+) T and innate NK lymphocytes independent of cognate antigen during microbial pathogen invasion. Immunity. 2012;37:549–562. doi: 10.1016/j.immuni.2012.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Serbina NV, Salazar-Mather TP, Biron CA, Kuziel WA, Pamer EG. TNF/iNOS-Producing Dendritic Cells Mediate Innate Immune Defense against Bacterial Infection. Immunity. 2003;19:59–70. doi: 10.1016/s1074-7613(03)00171-7. [DOI] [PubMed] [Google Scholar]
- 36.Berg RE, Crossley E, Murray S, Forman J. Memory CD8+ T cells provide innate immune protection against Listeria monocytogenes in the absence of cognate antigen. J Exp Med. 2003;198:1583–1593. doi: 10.1084/jem.20031051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Eash KJ, Means JM, White DW, Link DC. CXCR4 is a key regulator of neutrophil release from the bone marrow under basal and stress granulopoiesis conditions. Blood. 2009;113:4711–4719. doi: 10.1182/blood-2008-09-177287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ebe Y, Hasegawa G, Takatsuka H, Umezu H, Mitsuyama M, Arakawa M, Mukaida N, Naito M. The role of Kupffer cells and regulation of neutrophil migration into the liver by macrophage inflammatory protein-2 in primary listeriosis in mice. Pathol Int. 1999;49:519–532. doi: 10.1046/j.1440-1827.1999.00910.x. [DOI] [PubMed] [Google Scholar]
- 39.Semerad CL, Liu F, Gregory AD, Stumpf K, Link DC. G-CSF is an essential regulator of neutrophil trafficking from the bone marrow to the blood. Immunity. 2002;17:413–423. doi: 10.1016/s1074-7613(02)00424-7. [DOI] [PubMed] [Google Scholar]
- 40.Pittman K, Kubes P. Damage-associated molecular patterns control neutrophil recruitment. J Innate Immun. 2013;5:315–323. doi: 10.1159/000347132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thomas CJ, Schroder K. Pattern recognition receptor function in neutrophils. Trends Immunol. 2013;34:317–328. doi: 10.1016/j.it.2013.02.008. [DOI] [PubMed] [Google Scholar]
- 42.Southgate EL, He RL, Gao JL, Murphy PM, Nanamori M, Ye RD. Identification of Formyl Peptides from Listeria monocytogenes and Staphylococcus aureus as Potent Chemoattractants for Mouse Neutrophils. J Immunol. 2008;181:1429–1437. doi: 10.4049/jimmunol.181.2.1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu M, Chen K, Yoshimura T, Liu Y, Gong W, Wang A, Gao JL, Murphy PM, Wang JM. Formylpeptide receptors are critical for rapid neutrophil mobilization in host defense against Listeria monocytogenes. Sci Rep. 2012;2:786. doi: 10.1038/srep00786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gao JL, Lee EJ, Murphy PM. Impaired antibacterial host defense in mice lacking the N-formylpeptide receptor. J Exp Med. 1999;189:657–662. doi: 10.1084/jem.189.4.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Czuprynski CJ, Brown JF, Steinberg H, Carroll D. Mice lacking the murine interleukin-8 receptor homologue demonstrate paradoxical responses to acute and chronic experimental infection with Listeria monocytogenes. Microb Pathog. 1998;24:17–23. doi: 10.1006/mpat.1997.0169. [DOI] [PubMed] [Google Scholar]
- 46.Cacalano G, Lee J, Kikly K, Ryan AM, Pitts-Meek S, Hultgren B, Wood WI, Moore MW. Neutrophil and B cell expansion in mice that lack the murine IL-8 receptor homolog. Science. 1994;265:682–684. doi: 10.1126/science.8036519. [DOI] [PubMed] [Google Scholar]
- 47.Shuster DE, Kehrli ME, Jr, Ackermann MR. Neutrophilia in mice that lack the murine IL-8 receptor homolog. Science. 1995;269:1590–1591. doi: 10.1126/science.7667641. [DOI] [PubMed] [Google Scholar]
- 48.Brzoza-Lewis KL, Hoth JJ, Hiltbold EM. Type I interferon signaling regulates the composition of inflammatory infiltrates upon infection with Listeria monocytogenes. Cell Immunol. 2012;273:41–51. doi: 10.1016/j.cellimm.2011.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Arnaout MA. Structure and function of the leukocyte adhesion molecules CD11/CD18. Blood. 1990;75:1037–1050. [PubMed] [Google Scholar]
- 50.Wu H, Prince JE, Brayton CF, Shah C, Zeve D, Gregory SH, Smith CW, Ballantyne CM. Host Resistance of CD18 Knockout Mice against Systemic Infection with Listeria monocytogenes. Infect Immun. 2003;71:5986–5993. doi: 10.1128/IAI.71.10.5986-5993.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Miyamoto M, Emoto M, Emoto Y, Brinkmann V, Yoshizawa I, Seiler P, Aichele P, Kita E, Kaufmann SHE. Neutrophilia in LFA-1-Deficient Mice Confers Resistance to Listeriosis: Possible Contribution of Granulocyte-Colony-Stimulating Factor and IL-17. J Immunol. 2003;170:5228–5234. doi: 10.4049/jimmunol.170.10.5228. [DOI] [PubMed] [Google Scholar]
- 52.Rosen H, Gordon S, North RJ. Exacerbation of murine listeriosis by a monoclonal antibody specific for the type 3 complement receptor of myelomonocytic cells. Absence of monocytes at infective foci allows Listeria to multiply in nonphagocytic cells. J Exp Med. 1989;170:27–37. doi: 10.1084/jem.170.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hamada S, Umemura M, Shiono T, Tanaka K, Yahagi A, Begum MD, Oshiro K, Okamoto Y, Watanabe H, Kawakami K, Roark C, Born WK, O’Brien R, Ikuta K, Ishikawa H, Nakae S, Iwakura Y, Ohta T, Matsuzaki G. IL-17A Produced by γδ T Cells Plays a Critical Role in Innate Immunity against Listeria monocytogenes Infection in the Liver. J Immunol. 2008;181:3456–3463. doi: 10.4049/jimmunol.181.5.3456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Meeks KD, Sieve AN, Kolls JK, Ghilardi N, Berg RE. IL-23 is required for protection against systemic infection with Listeria monocytogenes. J Immunol. 2009;183:8026–8034. doi: 10.4049/jimmunol.0901588. [DOI] [PubMed] [Google Scholar]
- 55.Severa M, Islam SA, Waggoner SN, Jiang Z, Kim ND, Ryan G, Kurt-Jones E, Charo I, Caffrey DR, Boyartchuk VL, Luster AD, Fitzgerald KA. The transcriptional repressor BLIMP1 curbs host defenses by suppressing expression of the chemokine CCL8. J Immunol. 2014;192:2291–2304. doi: 10.4049/jimmunol.1301799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Czuprynski CJ, Brown JF. Purified human and recombinant murine interleukin-1 alpha induced accumulation of inflammatory peritoneal neutrophils and mononuclear phagocytes: possible contributions to antibacterial resistance. Microb Pathog. 1987;3:377–386. doi: 10.1016/0882-4010(87)90007-6. [DOI] [PubMed] [Google Scholar]
- 57.Czuprynski CJ, Brown JF. Recombinant murine interleukin-1 alpha enhancement of nonspecific antibacterial resistance. Infect Immun. 1987;55:2061–2065. doi: 10.1128/iai.55.9.2061-2065.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Czuprynski CJ, Brown JF, Young KM, Cooley AJ, Kurtz RS. Effects of murine recombinant interleukin 1 alpha on the host response to bacterial infection. J Immunol. 1988;140:962–968. [PubMed] [Google Scholar]
- 59.Kurlander RJ, Hoffman M, Kratz SS, Gates J. Comparison of the effects of IL-1 alpha and TNF-alpha on phagocyte accumulation and murine antibacterial immunity. Cell Immunol. 1989;123:9–22. doi: 10.1016/0008-8749(89)90264-5. [DOI] [PubMed] [Google Scholar]
- 60.Havell EA, Moldawer LL, Helfgott D, Kilian PL, Sehgal PB. Type I IL-1 receptor blockade exacerbates murine listeriosis. J Immunol. 1992;148:1486–1492. [PubMed] [Google Scholar]
- 61.Zheng H, Fletcher D, Kozak W, Jiang M, Hofmann KJ, Conn CA, Soszynski D, Grabiec C, Trumbauer ME, Shaw A, et al. Resistance to fever induction and impaired acute-phase response in interleukin-1 beta-deficient mice. Immunity. 1995;3:9–19. doi: 10.1016/1074-7613(95)90154-x. [DOI] [PubMed] [Google Scholar]
- 62.Rogers HW, Tripp CS, Schreiber RD, Unanue ER. Endogenous IL-1 is required for neutrophil recruitment and macrophage activation during murine listeriosis. J Immunol. 1994;153:2093–2101. [PubMed] [Google Scholar]
- 63.Dalrymple SA, Lucian LA, Slattery R, McNeil T, Aud DM, Fuchino S, Lee F, Murray R. Interleukin-6-deficient mice are highly susceptible to Listeria monocytogenes infection: correlation with inefficient neutrophilia. Infect Immun. 1995;63:2262–2268. doi: 10.1128/iai.63.6.2262-2268.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kopf M, Baumann H, Freer G, Freudenberg M, Lamers M, Kishimoto T, Zinkernagel R, Bluethmann H, Kohler G. Impaired immune and acute-phase responses in interleukin-6-deficient mice. Nature. 1994;368:339–342. doi: 10.1038/368339a0. [DOI] [PubMed] [Google Scholar]
- 65.Hoge J, Yan I, Janner N, Schumacher V, Chalaris A, Steinmetz OM, Engel DR, Scheller J, Rose-John S, Mittrucker HW. IL-6 controls the innate immune response against Listeria monocytogenes via classical IL-6 signaling. J Immunol. 2013;190:703–711. doi: 10.4049/jimmunol.1201044. [DOI] [PubMed] [Google Scholar]
- 66.Break TJ, Jun S, Indramohan M, Carr KD, Sieve AN, Dory L, Berg RE. Extracellular superoxide dismutase inhibits innate immune responses and clearance of an intracellular bacterial infection. J Immunol. 2012;188:3342–3350. doi: 10.4049/jimmunol.1102341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Helmy KY, Katschke KJ, Jr, Gorgani NN, Kljavin NM, Elliott JM, Diehl L, Scales SJ, Ghilardi N, van Lookeren Campagne M. CRIg: a macrophage complement receptor required for phagocytosis of circulating pathogens. Cell. 2006;124:915–927. doi: 10.1016/j.cell.2005.12.039. [DOI] [PubMed] [Google Scholar]
- 68.Arnett E, Vadia S, Nackerman CC, Oghumu S, Satoskar AR, McLeish KR, Uriarte SM, Seveau S. The pore-forming toxin listeriolysin O is degraded by neutrophil metalloproteinase-8 and fails to mediate Listeria monocytogenes intracellular survival in neutrophils. J Immunol. 2014;192:234–244. doi: 10.4049/jimmunol.1301302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhang H, Clemens RA, Liu F, Hu Y, Baba Y, Theodore P, Kurosaki T, Lowell CA. STIM1 calcium sensor is required for activation of the phagocyte oxidase during inflammation and host defense. Blood. 2014;123:2238–2249. doi: 10.1182/blood-2012-08-450403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lee SC, Ju SA, Pack HN, Heo SK, Suh JH, Park SM, Choi BK, Kwon BS, Kim BS. 4-1BB (CD137) is required for rapid clearance of Listeria monocytogenes infection. Infect Immun. 2005;73:5144–5151. doi: 10.1128/IAI.73.8.5144-5151.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lee SC, Ju SA, Sung BH, Heo SK, Cho HR, Lee EA, Kim JD, Lee IH, Park SM, Nguyen QT, Suh JH, Kim BS. Stimulation of the molecule 4-1BB enhances host defense against Listeria monocytogenes infection in mice by inducing rapid infiltration and activation of neutrophils and monocytes. Infect Immun. 2009;77:2168–2176. doi: 10.1128/IAI.01350-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Segal AW. The electron transport chain of the microbicidal oxidase of phagocytic cells and its involvement in the molecular pathology of chronic granulomatous disease. J Clin Invest. 1989;83:1785–1793. doi: 10.1172/JCI114083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Babior BM. NADPH oxidase. Curr Opin Immunol. 2004;16:42–47. doi: 10.1016/j.coi.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 74.Dinauer MC, Deck MB, Unanue ER. Mice lacking reduced nicotinamide adenine dinucleotide phosphate oxidase activity show increased susceptibility to early infection with Listeria monocytogenes. J Immunol. 1997;158:5581–5583. [PubMed] [Google Scholar]
- 75.Shiloh MU, MacMicking JD, Nicholson S, Brause JE, Potter S, Marino M, Fang F, Dinauer M, Nathan C. Phenotype of mice and macrophages deficient in both phagocyte oxidase and inducible nitric oxide synthase. Immunity. 1999;10:29–38. doi: 10.1016/s1074-7613(00)80004-7. [DOI] [PubMed] [Google Scholar]
- 76.LaCourse R, Ryan L, North RJ. Expression of NADPH Oxidase-Dependent Resistance to Listeriosis in Mice Occurs during the First 6 to 12 Hours of Liver Infection. Infect Immun. 2002;70:7179–7181. doi: 10.1128/IAI.70.12.7179-7181.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Endres R, Luz A, Schulze H, Neubauer H, Futterer A, Holland SM, Wagner H, Pfeffer K. Listeriosis in p47(phox−/−) and TRp55−/− mice: protection despite absence of ROI and susceptibility despite presence of RNI. Immunity. 1997;7:419–432. doi: 10.1016/s1074-7613(00)80363-5. [DOI] [PubMed] [Google Scholar]
- 78.Freeman JL, Lambeth JD. NADPH oxidase activity is independent of p47phox in vitro. J Biol Chem. 1996;271:22578–22582. doi: 10.1074/jbc.271.37.22578. [DOI] [PubMed] [Google Scholar]
- 79.West AP, I, Brodsky E, Rahner C, Woo DK, Erdjument-Bromage H, Tempst P, Walsh MC, Choi Y, Shadel GS, Ghosh S. TLR signalling augments macrophage bactericidal activity through mitochondrial ROS. Nature. 2011;472:476–480. doi: 10.1038/nature09973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Geng J, Sun X, Wang P, Zhang S, Wang X, Wu H, Hong L, Xie C, Li X, Zhao H, Liu Q, Jiang M, Chen Q, Zhang J, Li Y, Song S, Wang HR, Zhou R, Johnson RL, Chien KY, Lin SC, Han J, Avruch J, Chen L, Zhou D. Kinases Mst1 and Mst2 positively regulate phagocytic induction of reactive oxygen species and bactericidal activity. Nat Immunol. 2015;16:1142–1152. doi: 10.1038/ni.3268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Noubade R, Wong K, Ota N, Rutz S, Eidenschenk C, Valdez PA, Ding J, Peng I, Sebrell A, Caplazi P, DeVoss J, Soriano RH, Sai T, Lu R, Modrusan Z, Hackney J, Ouyang W. NRROS negatively regulates reactive oxygen species during host defence and autoimmunity. Nature. 2014;509:235–239. doi: 10.1038/nature13152. [DOI] [PubMed] [Google Scholar]
- 82.Hampton MB, Kettle AJ, Winterbourn CC. Involvement of superoxide and myeloperoxidase in oxygen-dependent killing of Staphylococcus aureus by neutrophils. Infect Immun. 1996;64:3512–3517. doi: 10.1128/iai.64.9.3512-3517.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bortolussi R, Vandenbroucke-Grauls CM, van Asbeck BS, Verhoef J. Relationship of bacterial growth phase to killing of Listeria monocytogenes by oxidative agents generated by neutrophils and enzyme systems. Infect Immun. 1987;55:3197–3203. doi: 10.1128/iai.55.12.3197-3203.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Aratani Y, Koyama H, Nyui S, Suzuki K, Kura F, Maeda N. Severe impairment in early host defense against Candida albicans in mice deficient in myeloperoxidase. Infect Immun. 1999;67:1828–1836. doi: 10.1128/iai.67.4.1828-1836.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.MacMicking JD, Nathan C, Hom G, Chartrain N, Fletcher DS, Trumbauer M, Stevens K, Xie QW, Sokol K, Hutchinson N, et al. Altered responses to bacterial infection and endotoxic shock in mice lacking inducible nitric oxide synthase. Cell. 1995;81:641–650. doi: 10.1016/0092-8674(95)90085-3. [DOI] [PubMed] [Google Scholar]
- 86.Cole C, Thomas S, Filak H, Henson PM, Lenz LL. Nitric oxide increases susceptibility of Toll-like receptor-activated macrophages to spreading Listeria monocytogenes. Immunity. 2012;36:807–820. doi: 10.1016/j.immuni.2012.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Muller M, Althaus R, Frohlich D, Frei K, Eugster HP. Reduced antilisterial activity of TNF-deficient bone marrow-derived macrophages is due to impaired superoxide production. Eur J Immunol. 1999;29:3089–3097. doi: 10.1002/(SICI)1521-4141(199910)29:10<3089::AID-IMMU3089>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 88.Edelson BT, Unanue ER. MyD88-Dependent but Toll-Like Receptor 2-Independent Innate Immunity to Listeria: No Role for Either in Macrophage Listericidal Activity. J Immunol. 2002;169:3869–3875. doi: 10.4049/jimmunol.169.7.3869. [DOI] [PubMed] [Google Scholar]
- 89.Arnold-Schrauf C, Dudek M, Dielmann A, Pace L, Swallow M, Kruse F, Kuhl AA, Holzmann B, Berod L, Sparwasser T. Dendritic cells coordinate innate immunity via MyD88 signaling to control Listeria monocytogenes infection. Cell Rep. 2014;6:698–708. doi: 10.1016/j.celrep.2014.01.023. [DOI] [PubMed] [Google Scholar]
- 90.Mosa A, Trumstedt C, Eriksson E, Soehnlein O, Heuts F, Janik K, Klos A, Dittrich-Breiholz O, Kracht M, Hidmark A, Wigzell H, Rottenberg ME. Nonhematopoietic cells control the outcome of infection with Listeria monocytogenes in a nucleotide oligomerization domain 1-dependent manner. Infect Immun. 2009;77:2908–2918. doi: 10.1128/IAI.01068-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Jeong YJ, Kang MJ, Lee SJ, Kim CH, Kim JC, Kim TH, Kim DJ, Kim D, Nunez G, Park JH. Nod2 and Rip2 contribute to innate immune responses in mouse neutrophils. Immunology. 2014;143:269–276. doi: 10.1111/imm.12307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Anand PK, Malireddi RK, Lukens JR, Vogel P, Bertin J, Lamkanfi M, Kanneganti TD. NLRP6 negatively regulates innate immunity and host defence against bacterial pathogens. Nature. 2012;488:389–393. doi: 10.1038/nature11250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Xia P, Wang S, Ye B, Du Y, Huang G, Zhu P, Fan Z. Sox2 functions as a sequence-specific DNA sensor in neutrophils to initiate innate immunity against microbial infection. Nat Immunol. 2015;16:366–375. doi: 10.1038/ni.3117. [DOI] [PubMed] [Google Scholar]
- 94.Lang KS, Lang PA, Meryk A, Pandyra AA, Boucher LM, Pozdeev VI, Tusche MW, Gothert JR, Haight J, Wakeham A, You-Ten AJ, McIlwain DR, Merches K, Khairnar V, Recher M, Nolan GP, Hitoshi Y, Funkner P, Navarini AA, Verschoor A, Shaabani N, Honke N, Penn LZ, Ohashi PS, Haussinger D, Lee KH, Mak TW. Involvement of Toso in activation of monocytes, macrophages, and granulocytes. Proc Natl Acad Sci USA. 2013;110:2593–2598. doi: 10.1073/pnas.1222264110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Harty JT, Bevan MJ. Specific immunity to Listeria monocytogenes in the absence of IFN gamma. Immunity. 1995;3:109–117. doi: 10.1016/1074-7613(95)90163-9. [DOI] [PubMed] [Google Scholar]
- 96.Huang S, Hendriks W, Althage A, Hemmi S, Bluethmann H, Kamijo R, Vilcek J, Zinkernagel RM, Aguet M. Immune response in mice that lack the interferon-gamma receptor. Science. 1993;259:1742–1745. doi: 10.1126/science.8456301. [DOI] [PubMed] [Google Scholar]
- 97.Berg RE, Cordes CJ, Forman J. Contribution of CD8+ T cells to innate immunity: IFN-gamma secretion induced by IL-12 and IL-18. Eur J Immunol. 2002;32:2807–2816. doi: 10.1002/1521-4141(2002010)32:10<2807::AID-IMMU2807>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 98.Berg RE, Crossley E, Murray S, Forman J. Relative contributions of NK and CD8 T cells to IFN-gamma mediated innate immune protection against Listeria monocytogenes. J Immunol. 2005;175:1751–1757. doi: 10.4049/jimmunol.175.3.1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Yin J, Ferguson TA. Identification of an IFN-gamma-producing neutrophil early in the response to Listeria monocytogenes. J Immunol. 2009;182:7069–7073. doi: 10.4049/jimmunol.0802410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Conlan JW. Neutrophils and tumour necrosis factor-alpha are important for controlling early gastrointestinal stages of experimental murine listeriosis. J Med Microbiol. 1997;46:239–250. doi: 10.1099/00222615-46-3-239. [DOI] [PubMed] [Google Scholar]
- 101.Rothe J, Lesslauer W, Lotscher H, Lang Y, Koebel P, Kontgen F, Althage A, Zinkernagel R, Steinmetz M, Bluethmann H. Mice lacking the tumour necrosis factor receptor 1 are resistant to TNF-mediated toxicity but highly susceptible to infection by Listeria monocytogenes. Nature. 1993;364:798–802. doi: 10.1038/364798a0. [DOI] [PubMed] [Google Scholar]
- 102.Pasparakis M, Alexopoulou L, Episkopou V, Kollias G. Immune and inflammatory responses in TNF alpha-deficient mice: a critical requirement for TNF alpha in the formation of primary B cell follicles, follicular dendritic cell networks and germinal centers, and in the maturation of the humoral immune response. J Exp Med. 1996;184:1397–1411. doi: 10.1084/jem.184.4.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Pfeffer K, Matsuyama T, Kundig TM, Wakeham A, Kishihara K, Shahinian A, Wiegmann K, Ohashi PS, Kronke M, Mak TW. Mice deficient for the 55 kd tumor necrosis factor receptor are resistant to endotoxic shock, yet succumb to L. monocytogenes infection. Cell. 1993;73:457–467. doi: 10.1016/0092-8674(93)90134-c. [DOI] [PubMed] [Google Scholar]
- 104.Grivennikov SI, Tumanov AV, Liepinsh DJ, Kruglov AA, Marakusha BI, Shakhov AN, Murakami T, Drutskaya LN, Forster I, Clausen BE, Tessarollo L, Ryffel B, Kuprash DV, Nedospasov SA. Distinct and nonredundant in vivo functions of TNF produced by T cells and macrophages/neutrophils: protective and deleterious effects. Immunity. 2005;22:93–104. doi: 10.1016/j.immuni.2004.11.016. [DOI] [PubMed] [Google Scholar]
- 105.Leist M, Gantner F, Jilg S, Wendel A. Activation of the 55 kDa TNF receptor is necessary and sufficient for TNF-induced liver failure, hepatocyte apoptosis, and nitrite release. J Immunol. 1995;154:1307–1316. [PubMed] [Google Scholar]
- 106.Leist M, Gantner F, Bohlinger I, Germann PG, Tiegs G, Wendel A. Murine hepatocyte apoptosis induced in vitro and in vivo by TNF-alpha requires transcriptional arrest. J Immunol. 1994;153:1778–1788. [PubMed] [Google Scholar]
- 107.Czuprynski CJ, Brown JF, Wagner RD, Steinberg H. Administration of antigranulocyte monoclonal antibody RB6-8C5 prevents expression of acquired resistance to Listeria monocytogenes infection in previously immunized mice. Infect Immun. 1994;62:5161–5163. doi: 10.1128/iai.62.11.5161-5163.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Narni-Mancinelli E, Soudja SM, Crozat K, Dalod M, Gounon P, Geissmann F, Lauvau G. Inflammatory monocytes and neutrophils are licensed to kill during memory responses in vivo. PLoS Pathog. 2011;7:e1002457. doi: 10.1371/journal.ppat.1002457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Narni-Mancinelli E, Campisi L, Bassand D, Cazareth J, Gounon P, Glaichenhaus N, Lauvau G. Memory CD8+ T cells mediate antibacterial immunity via CCL3 activation of TNF/ROI+ phagocytes. J Exp Med. 2007;204:2075–2087. doi: 10.1084/jem.20070204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Soudja SM, Chandrabos C, Yakob E, Veenstra M, Palliser D, Lauvau G. Memory-T-cell-derived interferon-gamma instructs potent innate cell activation for protective immunity. Immunity. 2014;40:974–988. doi: 10.1016/j.immuni.2014.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Chaturvedi V, Ertelt JM, Jiang TT, Kinder JM, Xin L, Owens KJ, Jones HN, Way SS. CXCR3 blockade protects against Listeria monocytogenes infection-induced fetal wastage. J Clin Invest. 2015;125:1713–1725. doi: 10.1172/JCI78578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ordonez-Rueda D, Jonsson F, Mancardi DA, Zhao W, Malzac A, Liang Y, Bertosio E, Grenot P, Blanquet V, Sabrautzki S, de Angelis MH, Meresse S, Duprez E, Bruhns P, Malissen B, Malissen M. A hypomorphic mutation in the Gfi1 transcriptional repressor results in a novel form of neutropenia. Eur J Immunol. 2012;42:2395–2408. doi: 10.1002/eji.201242589. [DOI] [PubMed] [Google Scholar]