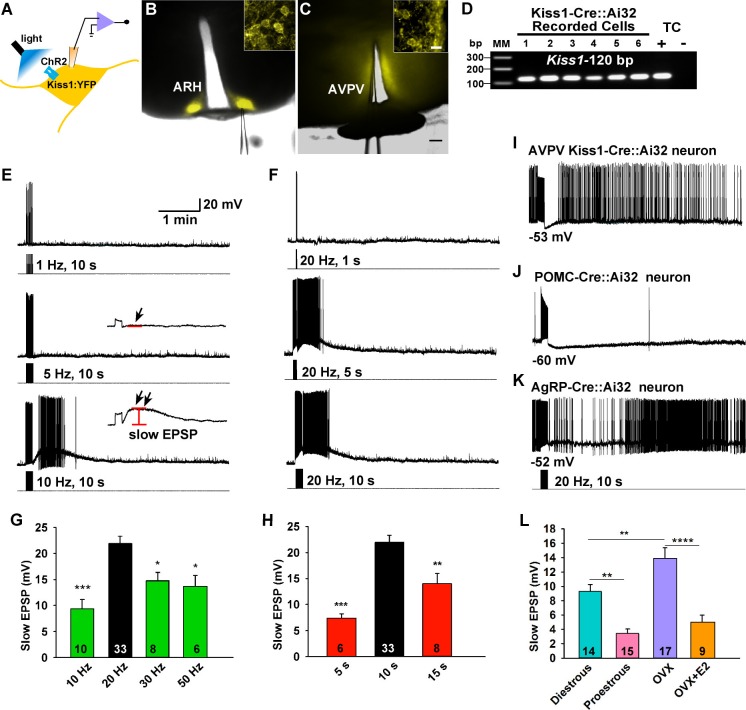
Figure 2. Slow excitatory postsynaptic potential (slow EPSP) is frequency and duration dependent.
(A) Illustration of experimental approach. (B–C), Overlay of epifluorescence (YFP) and differential interference contrast (DIC) images during whole-cell recording (pipette tip can be seen) from Kiss1 neurons in ARH (B) and AVPV (C) brain slices from a Kiss1-Cre::Ai32 mouse (scale bar = 200 µm). (B,C) Insets show high power magnification of labeled cells in each area (scale bar = 10 µm). ARH, hypothalamic arcuate nucleus; AVPV, anteroventral periventricular nucleus. (D) Confirmation of Kiss1 expression in YFP-expressing neurons from Kiss1-Cre::Ai32. Representative gel illustrating single-cell RT-PCR identification of Kiss1 mRNA following whole-cell recording in ChR2-YFP neurons. RNA extracted from the medial basal hypothalamic tissue control (TC) was included as positive (+, with RT) and negative (-, without RT) controls. MM, molecular marker. (E) Slow EPSP induced by a 10-s photostimulation (light intensity 0.9 mW and pulse duration, 10 ms) with varied frequencies in the same neuron. The insets show the measurement of slow EPSP after low-pass filtering. 1–5 Hz photostimulation did not induce any post-stimulus depolarization (arrow, middle trace); but ≥10 Hz stimulation generated a significant post-stimulus depolarization (double arrow, lower trace). (F), Examples of synaptic responses induced by a train of stimuli (0.9 mW, 10 ms pulse-width) delivered at 20 Hz with varied duration in the same Kiss1ARH neuron. (G–H), Bar graphs summarizing slow EPSP responses induced by a train of stimulation at 10, 20, 30 and 50 Hz with duration of 10 s (G) and by a 20 Hz-stimulation delivered at 5, 10 and 15 s (H), current-clamped to -70 mV. The slow EPSP was larger when induced by 10-s and 20 Hz photostimulation (one-way ANOVA, effect of treatment, F(3, 53) = 9.912, p<0.0001 (G); F(2, 44) = 12.69, p<0.0001 (H); Newman-Keuls’s Multiple-comparison test, ***, **, * indicates p<0.005, 0.01 and 0.05, respectively. (I–K), Slow EPSP is unique to arcuate Kiss1 neurons. (I) Photostimulation induced auto-inhibition in AVPV Kiss1 neuron, (J) ARH POMC neuron and (K) AgRP neuron from Kiss1-Cre::Ai32, POMC-Cre::Ai32 and AgRP-Cre::Ai32 mice, respectively. (L) Light evoked slow EPSP in Kiss1ARH neurons was reduced by E2 treatment and varied during the ovulatory cycle. Bar graphs summarizing slow EPSP responses induced by 20 Hz, 10 s photostimulation in slices obtained from diestrous or proestrous females, or OVX females treated with either oil vehicle or E2 that had received injection of AAV-DIO-ChR2:YFP into ARH. Slow EPSP was larger in low estrogen states (one-way ANOVA, effect of treatment, F (3, 51) = 15.43, p<0.0001; Newman-Keuls’s Multiple-comparison test. ****, ** indicates p<0.001 and 0.01, respectively.