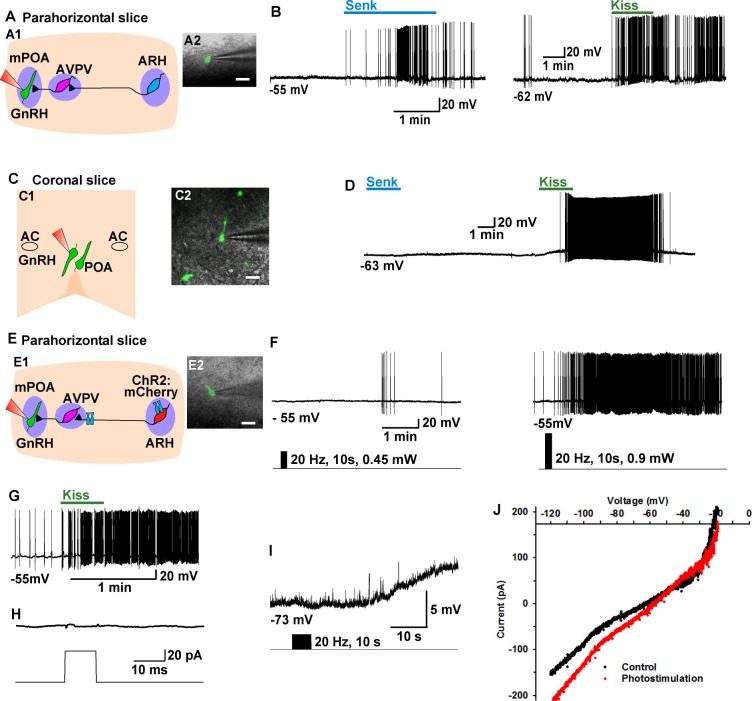
Figure 9. Tacr3 agonist senktide excites GnRH neurons indirectly through ARH Kiss1 neurons, and a slow EPSP in GnRH neurons is evoked with photoactivation of ChR2 Kiss1ARH neurons.
(A) Schematic drawing of the Kiss1ARH, Kiss1AVPV/PeN and GnRH neuronal circuit (A1) and a high power image (A2) of a parahorizontal slice from a GnRH-EGFP mouse illustrating whole-cell recording of GnRH neuron. Scale bar = 20 µM. (B) Representative traces showing that perfusing the slice with senktide (1 µM) induced depolarization and increased firing in a GnRH neuron (left), which was mimicked by perfusion with kisspeptin (10 nM, right). (C) Schematic drawing (C1) and highpower image (C2) of a coronal slice through the POA from a GnRH-EGFP mouse illustrating whole-cell recording of GnRH neuron. (D) Representative trace showing that perfusing the slice with senktide (1 µM) did not depolarize or increase firing of a GnRH neuron, but kisspeptin could excite the same POA GnRH neuron. E, Schematic drawing of the Kiss1ARH, Kiss1AVPV/PeN and GnRH neuronal circuit (E1) and a high power image (E2) of a parahorizontal slice from a bilateral arcuate AAV-DIO-ChR2:mCherry injected Kiss1-Cre::GnRH-EGFP mouse illustrating whole-cell recording of GnRH neuron. Scale bar, 20 µM (E2). (F–G) High-frequency photoactivation of ChR2-labeled ARH neurons with low (0.45 mW, left panel) and higher (0.9 mW, right panel) intensities evoked slow EPSPs and increased firing in the same GnRH neuron (F) which was mimicked by perfusion with kisspeptin (10 nM) (G) but low-frequency photostimulation (0.5 Hz) did not evoke a fast EPSC in this GnRH neuron (H). (I–J) High-frequency photoactivation of ChR2 evoked a slow EPSP (I) in another GnRH neuron, and the I-V relationship before and during the peak response from the same cell indicated that the reversal potential of the nonselective cation current was ~ -40 mV (J). AC, anterior commissure; ARH, hypothalamic arcuate nucleus; AVPV, anteroventral periventricular nucleus; mPOA, medial preoptic area.