Abstract
The thymus is critical for mounting an effective immune response and maintaining health. However, epidemiologic studies characterizing thymic function in the population setting are lacking. Using data from 263 adults in the Detroit Neighborhood Health Study, we examined thymic function as measured by the number of signal joint T-cell receptor excision circles (sjTREC) and assessed associations with established indicators of physiological health. Overall, increasing age and male gender were significantly associated with reduced thymic function. Adjusting for covariates, individuals with elevated levels of the pro-inflammatory biomarkers C-reactive protein (β: −0.50 [95% CI: −0.82, −0.18] for moderate elevation; β: −0.29 [95% CI: −0.59, 0.00] for high elevation) and interleukin-6 (β: −0.60 [95% CI: −0.92, −0.28] for moderate elevation; β: −0.43 [95% CI: −0.77, −0.08] for severe elevation) also had lower thymic function. Compared to individuals with a BMI <25, individuals who were overweight (β: 0.36 [95% CI: 0.07, 0.64]) or obese (β: 0.27 [95% CI: −0.03, 0.56]) had higher thymic function. Differences by self-rated health were not statistically significant. Our findings underscore demographic- and health-related gradients in thymic function among adult residents of Detroit, suggesting thymic function may be an important biomarker of health status in adults at the population level.
Keywords: Aging, Body mass index, Detroit, C-reactive protein, Immunity, Immunosenescence, Interleukin-6, Thymic function
Introduction
Thymic function has recently emerged as a significant predictor of mortality in adults independent of other immunological markers, suggesting that thymic function may influence overall health outcomes (Ferrando-Martinez et al. 2013). The thymus is a specialized organ that plays a vital role in the maturation of T cells, which are critical for mounting an effective immune response in humans. Thymic involution, or the shrinking of the thymus with age, is one of the most prominent and ubiquitous changes in immune function over the life course (Palmer 2013). Until recently, it was generally accepted that the process of thymic involution was completed and that the thymus ceased to function by early adulthood. Although a reduction in naïve T cells derived from the thymus does occur more rapidly in early life, it is now established that the thymus continues to play a prominent role in the supply of naïve T cells throughout adulthood, including in old age (Poulin et al. 1999, Douek et al. 1998).
While thymic atrophy is likely to have few immediate health consequences for young individuals with an otherwise healthy immune system, reduced thymic output of naïve T cells in older adults is likely to compound other immune system declines. Indeed, reduced thymic function has been linked to increased susceptibility to infection, autoimmune disease, and cancer (Lynch et al. 2009). Among individuals infected with HIV, a higher level of thymic function is known to contribute to immune reconstitution and associated health improvements following initiation of antiretroviral therapy (Douek and Koup 2000, Douek et al. 1998, Harris et al. 2005, Kolte 2013, Ye, Kirschner, and Kourtis 2004).
Despite the growing evidence supporting an important role of adult thymic function to health outcomes, relatively little is known about the distribution of thymic function at the population level as there are no epidemiological studies, of which we are aware, that have characterized thymic function in the population setting. Using data drawn from a population-based sample of adults living in Detroit, we examined the distribution of thymic function and assessed associations with established markers of physiological health, including the pro-inflammatory biomarkers C-reactive protein (CRP) and interleukin-6 (IL-6), body mass index (BMI), and self-rated health status. We measured thymic function as the number of signal joint T-cell receptor excision circles (sjTREC), nonreplicated extrachromosomal DNA by-products of TCR gene rearrangements that are present in recently produced T cells (Lynch and Sempowski 2013, Douek et al. 1998). Although sjTREC do not directly contribute to the immune response, they serve as a key indicator of the supply of recent thymic emigrants and have been widely shown to decline significantly with age (Douek et al. 1998, Hazenberg et al. 2003, Lynch and Sempowski 2013, Mitchell, Lang, and Aspinall 2010). Thus, sjTREC represent one of the most established biomarkers of thymic function currently available in human populations. Another important advantage of sjTREC quantification for assessing thymic function in larger population-based samples such as ours is that, unlike flow cytometry for immunophenotyping, sjTREC quantification does not require viable cells and is thus well-suited for assessing thymic function in appropriately stored blood samples.
Methods
Study population
We used data from the Detroit Neighborhood Health Study (DNHS), a longitudinal study of predominantly African American adults aged 18 years or older living in Detroit, Michigan with a total of five annual surveys conducted between 2008 and 2013. DNHS participants were selected via a two-stage area probability sample of households within the Detroit city limits. For each household, one adult was randomly selected to participate in a 40-minute telephone survey with questions on participants’ sociodemographics, neighborhood characteristics, physical and mental health, social support, exposure to stressful events, and substance use behaviors. The survey instruments included reliable scales that have been validated or used in comparable research in the past, and the survey was pilot tested before each study wave (Horesh et al. 2014, Goldmann et al. 2011, Uddin et al. 2010). Surveys were conducted via computer-assisted telephone interviewing (CATI).
In Wave 1 (2008-2009), 1547 adult participants were recruited from the Detroit population to participate in the telephone survey. Detailed information on the sampling frame, recruitment procedures, and sample characteristics have been published previously (Uddin et al. 2010). Wave 1 participants were re-contacted in Wave 2 (2009-2010) for a follow-up interview. To increase sample size, a supplemental sample of 534 new participants was drawn via the same sampling technique used in Wave 1, providing a total of 2081 participants who have ever completed a survey. After Wave 2, no new participants were added, but all existing participants were re-contacted each year to participate. In addition to the telephone survey, participants had the option to also contribute a venous blood sample at their home. All participants who provided an initial blood sample (n=775) and again provided a blood sample in Wave 4 (2011-2012) of the study (n=317) —when thymic function was assessed—were eligible for inclusion in the analysis. Individuals whose samples yielded insufficient DNA to quantify thymic function and those without inflammatory marker test results were excluded from the analysis, resulting in a final sample size of 263 individuals. At the baseline survey, the 263 individuals who were included in the analysis sub-sample were slightly older than the full 2081 sample (median age 58 versus 53 years), and slightly less likely to be non-Hispanic Black/African American (80% versus 85%). Other socio-demographic characteristics, including gender and educational attainment, were similar between the full and subsamples.
Ethics Approval
All participants provided informed consent for participation and the study was approved by the University of Michigan Institutional Review Board and the University of North Carolina Institutional Review Board.
Measures
Thymic function quantification
Participant DNA were extracted from venous whole blood samples, frozen and stored at −70 C°, and shipped on dry ice to the Laboratory of Immunovirology at the University of Seville, Spain for thymic function quantification. Thymic function was measured by the number of signal joint T-cell receptor excision circles (sjTREC) per million whole blood cells. Molecular sjTREC were analyzed in genomic DNA in a two-round quantitative PCR (qPCR) protocol as previously described (Ferrando-Martinez et al. 2010, Ferrando-Martinez et al. 2013). Briefly, after a first round PCR [5 min at 95 °C, 21 amplification cycles (95 °C for 20 s; 57 °C for 45 s; 72 °C for 30 s), 5 min at 72°C], amplicons were amplified in a second PCR round using a LightCycler® 480 system (Roche, Manheim, Germany). For the qPCR, six microliters of a 1/10 mixed dilution of the first round PCR was amplified in a 20 μL final volume using Forster Resonance Energy Transfer (FRET) specific probes previously described (Franco et al. 2002). sjTREC abundance was normalized to cell number by amplification of β-globin with the GH20 and PC04 primers (Bauer et al. 1991). Standard curves were generated as previously described (Ferrando-Martinez et al. 2010) and run together with the samples in each experiment. In statistical analyses, sjTREC quantification was treated as a continuous outcome and natural log-transformed to approximate a normal distribution.
Ascertainment of physiological health
Four measures of physiological health were assessed, including serum levels of the pro-inflammatory biomarkers C-reactive protein (CRP) and interleukin-6 (IL-6), body mass index (BMI), and self-rated health status. CRP and IL-6 were assessed in Waves 1 and 2 of DNHS; the present analysis uses the most recently measured value. CRP concentration was measured in serum using the CRPUltra Wide Range Reagent Kit (Genzyme, USA) and following the manufacturer's recommended protocols. CRP values were categorized into three groups based on established clinical cut points for heart disease risk: <1, 1-3, and >3 mg/L (MedlinePlus [Internet]). IL-6 concentration was measured using the QuantiGlo Human IL-6 sandwich enzyme immunoassay kit (R&D Systems, USA) and following the manufacturer's recommended protocol. As there are no established clinical cut points for IL-6, IL-6 levels were categorized based on the study population distribution with cut points at the lower and upper quartile: <1.5, 1.5-4.3, and >4.3 pg/mL. CRP and IL-6 values that fell below the limit of detection (0.05 for CRP and 0.50 for IL-6) were assigned values of 0.025 and 0.25, respectively, as done in prior studies (Simanek et al. 2014). BMI was assessed in participants’ home at the time of the Wave 4 blood draw and was categorized base on established clinical cut-points: <18.5 (underweight), 18.5 to <25 (normal or healthy weight), 25 to <30 (overweight), or ≥30 (obese) (Sipahi et al. 2014). The lowest two categories were collapsed because only 7 individuals had a BMI <18.5. Self-rated health was assessed during the Wave 4 telephone interview and categorized as: excellent, very good or good, and fair or poor.
Assessment of covariates
Potential confounding factors of the associations between the four markers of physiological health and thymic function were assessed via a directed acyclic graph (Greenland, Pearl, and Robins 1999) and included the variables age, gender, race/ethnicity, socio-economic status, and cigarette smoking status for all exposure-outcome associations. Additional potential confounding variables differed for each exposure-outcome association: BMI and acute illness were additionally considered as potential confounders of the association between self-rated health and thymic function; BMI, acute illness, and self-rated health were additionally considered as potential confounders of the association between IL-6 and thymic function; and BMI, acute illness, self-rated health, and IL-6 were additionally considered as potential confounders of the association between CRP and thymic function. All covariates other than BMI and IL-6 were evaluated in the Wave 4 telephone interview. Age was modeled continuously in years and gender was assessed dichotomously as female or male. Race/ethnicity was assessed in the survey as Asian, Black or African American, American Indian or Alaskan Native, Native Hawaiian or Other Pacific Islander, White, Hispanic, or other; to increase precision, we dichotomized race/ethnicity in the present analysis as non-Hispanic Black/African American or other. Socio-economic status was operationalized as educational attainment, which was dichotomized as less than or equal to a high school education or more than a high school education. Cigarette smoking status was categorized as current, former, or never. Individuals were considered acutely ill if they reported experiencing any of the following eight conditions in the prior 60 days: flu, cold, pneumonia, vomiting, diarrhea, runny nose, cough, or sore throat.
Statistical analysis
Statistical analyses were conducted in SAS 9.4 (SAS Institute, Inc., Cary, North Carolina). To make inference to the Detroit population, data were weighted to account for the survey design and to balance the sub-sample to the city of Detroit. Control totals were constructed from the 2008-2010 American Community Survey (ACS) Public Use Microdata Sample (PUMS) for the following eight variables: age, gender, education, marital status, household size, tenure status (i.e., own residence or rent), race/ethnicity, and type of telephone survey (e.g., cell phone only or unlisted landline). Sample balancing was implemented using the IHB raking macro in SAS and the final raked weights were trimmed using the Individual and Global Cap Value (IGCV) method (Izrael, Hoaglin, and Battaglia 2000, Izrael, Battaglia, and Frankel 2009).
Demographics and clinical characteristics of the study population were evaluated using standard descriptive statistics, including medians and interquartile ranges (IQRs) for continuous variables and counts and proportions for categorical variables. We evaluated gender differences in thymic function by comparing age-specific and age-adjusted means between females and males. Next, we separately estimated the associations between the four measures of physiological health and thymic function using OLS linear regression. Three models were assessed: the first model adjusted only for age and the second model additionally adjusted for gender and race/ethnicity. The covariates included in the third model varied by exposure: the third model for BMI additionally adjusted for education and smoking status; the third model for self-rated health additionally adjusted for education, smoking status, acute illness, and BMI; the third model for CRP additionally adjusted for education, smoking status, acute illness, self-rated health, BMI, and IL-6; and the third model for IL-6 additionally adjusted for education, smoking status, acute illness, self-rated health, and BMI. We conducted a complete case analysis, excluding observations with missing values for covariates (2% in the full model). All tests of statistical significance were 2-sided and the threshold for statistical significance was P < 0.05.
Results
The weighted distributions of participants’ sociodemographic and biomedical characteristics are shown in Table 1. Participants were a median of 48 years of age (IQR: 38-62); approximately half (49%) were female and the majority (88%) reported their race/ethnicity as Black or African American. Approximately half of the participants (53%) had at most a high school education and 33% reported that they currently smoked cigarettes. Participants had a median BMI of 29 (IQR: 26-36), a median CRP level of 2.1 (0.5-5.1), and a median IL-6 level of 2.7 (1.4-4.4). 38% reported their health status as fair or poor.
Table 1.
Weighted Sample Characteristics (N=263), Detroit Neighborhood Health Study, 2008-2012.
| Distribution | |
|---|---|
| Age in years, median (IQR) | 48 (38-62) |
| Gender, N (%) | |
| Female | 130 (49) |
| Male | 133 (51) |
| Race/Ethnicity, N (%) | |
| Non-Hispanic Black/African American | 232 (88) |
| Other | 31 (12) |
| Education, N (%) | |
| ≤High school | 125 (48%) |
| >High school | 138 (52%) |
| Cigarette Smoking status, N (%) | |
| Never smoked | 98 (37) |
| Formerly smoked | 79 (30) |
| Currently smokes | 86 (33) |
| Body Mass Index, N (%) | |
| <25 | 60 (23) |
| 25 to <30 | 86 (33) |
| ≥30 | 111 (43) |
| Self-rated health, N (%) | |
| Excellent | 33 (13) |
| Very Good/Good | 130 (50) |
| Fair/Poor | 99 (38) |
| C-reactive protein, N (%) | |
| <1 mg/L | 110 (42) |
| 1 to 3 mg/L | 56 (21) |
| >3 mg/L | 96 (37) |
| Interleukin-6, N (%) | |
| <1.5 pg/mL | 68 (26) |
| 1.5-4.3 pg/mL | 129 (49) |
| >4.3 pg/mL | 66 (25) |
Abbreviations: IQR, interquartile range
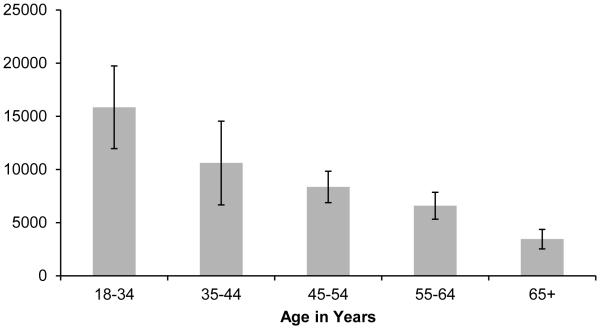
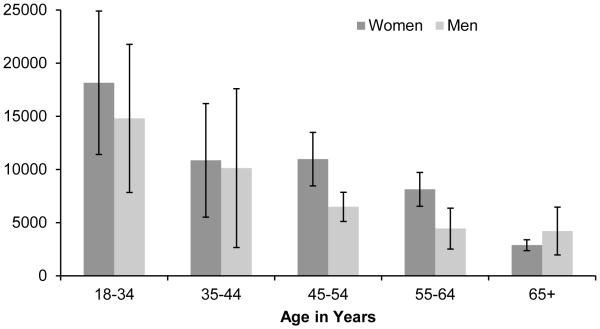
We observed a statistically significant (p<0.0001) decline in thymic function with age, with each one year increase in age associated with a −0.039 [95% CI: −0.045, −0.034] log-unit decrease in sjTREC per million whole blood cells. Figure 1 shows the mean number of sjTREC per million whole blood cells stratified by age group; the mean number of sjTREC was 15,842 in individuals 18-34 years of age, 10,605 in individuals 35-44 years of age, 8,362 in individuals 45-54 years of age, 6,584 in individuals 55-64 years of age, and 3,447 in individuals 65 years of age and older. Overall, women had a statistically higher number of log-sjTREC than men adjusting for age (β: 0.33 [95% CI: 0.13, 0.54]). However, this difference did not hold across all age groups (see Figure 2).
Figure 1.
Mean Thymic Output (sjTREC per Million Whole Blood Cells) by Age (N=263), Detroit Neighborhood Health Study, 2008-2012.
*Error bars represent 95% confidence intervals.
Figure 2.
Mean Thymic Output (sjTREC per Million Whole Blood Cells) by Age and Gender (N=263), Detroit Neighborhood Health Study, 2008-2012.
*Error bars represent 95% confidence intervals.
The covariate-adjusted associations between the four markers of physiological health and thymic function are shown in Table 2. After adjusting for the full set of covariates, we observed that individuals who had moderately elevated levels of CRP (1-3 mg/L) had a −0.50 (95% CI: −0.82, −0.18) log-unit decrease in the number of sjTREC per million whole blood cells and individuals who had highly elevated levels of CRP (>3 mg/L) had a −0.29 (95% CI: −0.59, 0.00) log-unit decrease in the number of sjTREC per million whole blood cells compared to individuals with CRP levels in the normal range (<1 mg/L). Similar associations were observed for IL-6; individuals who had IL-6 levels that fell within the interquartile range (1.5-4.3 pg/mL) had a −0.60 (95% CI: −0.92, −0.28) log-unit decrease in the number of sjTREC per million whole blood cells and individuals who had IL-6 levels above the upper quartile range (>4.3 pg/mL) had a −0.43 (95% CI: −0.77, −0.08) log-unit decrease in the number of sjTREC per million whole blood cells compared to individuals with IL-6 levels below the lower quartile range (<1.5 pg/mL) after adjustment for potential confounders. A weaker and non-significant association was observed between self-rated health and thymic function in adjusted models. Individuals who reported their health status was very good or good (β: −0.27 [95% CI: −0.63, 0.09]) and individuals who reported their health status was fair or poor (β: −0.12 [95% CI: −0.54, 0.30]) had reduced thymic function compared to those who reported being in excellent health. Individuals who were overweight (BMI 25 to <30) or obese (BMI ≥30) appeared to have improved thymic function compared to individuals with a BMI <25, with regression coefficients of 0.36 (95% CI: 0.07, 0.64) and 0.27 (95% CI: −0.03, 0.56), respectively. Regression coefficients for all models are shown in Supplementary Tables 1-4.
Table 2.
Regression Coefficients (β) and 95% Confidence Intervals for the Associations Between Markers of Physiological Health and Thymic Output (log sjTREC per Million Whole Blood Cells), Detroit Neighborhood Health Study, 2008-2012.
| β (95% Confidence Interval) | |||
|---|---|---|---|
|
|
|||
| Model 1 | Model 2 | Model 3 | |
| Body mass index a | |||
| <25 | REF | REF | REF |
| 25 to <30 | 0.40 (0.12, 0.68) | 0.38 (0.10, 0.66) | 0.36 (0.07, 0.64) |
| ≥30 | 0.45 (0.18, 0.71) | 0.31 (0.02, 0.59) | 0.27 (−0.03, 0.56) |
| P=0.0030 | P=0.0263 | P=0.0462 | |
| Self-rated health b | |||
| Excellent | REF | REF | REF |
| Very good/Good | −0.13 (−0.46, 0.21) | −0.27 (−0.61, 0.07) | −0.27 (−0.63, 0.09) |
| Fair/Poor | 0.04 (−0.32, 0.40) | −0.11 (−0.48, 0.26) | −0.12 (−0.54, 0.30) |
| P=0.3082 | P=0.1762 | P=0.1854 | |
| C-reactive protein c | |||
| <1 | REF | REF | REF |
| 1 to 3 | −0.29 (−0.57, −0.01) | −0.34 (−0.61, −0.06) | −0.50 (−0.82, −0.18) |
| >3 | −0.07 (−0.30, 0.17) | −0.20 (−0.44, 0.04) | −0.29 (−0.59, 0.00) |
| P=0.1208 | P=0.0424 | P=0.0082 | |
| Interleukin-6 d | |||
| <1.5 | REF | REF | REF |
| 1.5-4.3 | −0.03 (−0.28, 0.22) | −0.29 (−0.56, −0.01) | −0.60 (−0.92, −0.28) |
| >4.3 | 0.03 (−0.26, 0.32) | −0.24 (−0.56, 0.07) | −0.43 (−0.77, −0.08) |
| P=0.8989 | P=0.1288 | P=0.0014 | |
Model 1 is adjusted for age; Model 2 is additionally adjusted for gender and race; and Model 3 is additionally adjusted for education, and smoking status.
Model 1 is adjusted for age; Model 2 is additionally adjusted for gender and race; and Model 3 is additionally adjusted for education, smoking status, acute illness, and BMI.
Model 1 is adjusted for age; Model 2 is additionally adjusted for gender and race; and Model 3 is additionally adjusted for education, smoking status, acute illness, self-rated health, BMI, and IL-6.
Model 1 is adjusted for age; Model 2 is additionally adjusted for gender and race; and Model 3 is additionally adjusted for education, smoking status, acute illness, self-rated health, and BMI.
Discussion
In the present study, we assessed the distribution of thymic function as measured by the number of sjTREC per million whole blood cells in a population-based sample of adults living in Detroit, MI. We found that increasing age and female gender were significantly associated with a reduction in thymic function. Moreover, our results suggested that several indicators of poor health status, including BMI and elevated levels of the pro-inflammatory biomarkers CRP and IL-6, are also significantly associated with reduced thymic function after controlling for potential confounding factors. Taken together, these findings provide strong evidence from a population-based study that thymic function is an important biomarker of health status in adults at the population level.
To the best of our knowledge, the present paper also provides the first age- and gender-stratified estimates of thymic function from a population-based sample of adults, which could potentially be used as reference values in future studies or in the clinical setting. Our findings support the conclusion from prior smaller studies based on samples obtained from patients who had undergone cardiac surgery or suffered brain death, which showed that the thymus is indeed active in adults and continues to contribute to the pool of naïve T cells (Ferrando-Martinez et al. 2009, Jamieson et al. 1999). In a sample of 195 healthy Dutch volunteers, Zubakov et al. found that thymic function as measured by the number of sjTREC explained over 80% of the age variation and thus proposed sjTREC quantification as a potential molecular method for accurately estimating human chronological age in the forensic context (Zubakov et al. 2010).
We also observed that females had significantly higher thymic function than males, although the difference was not statistically significant across all age groups. Interestingly, we observed that women had lower thymic function than males among those 65 years of age and older. Although not statistically significant, it is possible that this reverse trend is attributable to a survivor bias whereby healthy males are overrepresented in the oldest age group. As prior studies have also demonstrated higher thymic function for women compared to men (Yoshida et al. 2014, Zubakov et al. 2010, Lorenzi et al. 2008) and gender differences have been demonstrated for other markers of immune status and decline (Barrett and Richardson 2011, Pennell, Galligan, and Fish 2012, Yan et al. 2010), future studies with larger sample sizes are warranted to further explore whether thymic function differs between men and women at the population level. Longitudinal studies may also untangle whether these potential differences are sustained across the life course.
Our findings that levels of the pro-inflammatory cytokines IL-6 and CRP are associated with poorer thymic function are consistent with prior studies in animals and humans that have identified these cytokines as thymosuppressive agents. Sempowski et al. observed that several members of the IL-6 cytokine gene family, including IL-6, acutely involute the thymus when injected into young, healthy mice and that IL-6 gene markers are also more prevalent in older human thymus tissue (Gruver and Sempowski 2008, Sempowski et al. 2000). Moreover, using data obtained from a human cohort of atomic bomb survivors, Yoshida et al. found that higher CRP levels are associated with reduced thymic function (Yoshida et al. 2014). Proinflammatory cytokines thus appear to play a key role in thymic involution associated with aging. However, as described in the methods section, thymic function and the proinflammatory biomarkers were assessed cross-sectionally in different study waves. It is therefore not possible to draw firm conclusions about the causal mechanisms underlying the statistical associations between thymic function and these biomarkers from the present study. These mechanisms are likely bi-directional to some degree and therefore the observed associations warrant further investigation in longitudinal studies. In addition, our results for the associations between proinflammatory cytokines and thymic function did not demonstrate a dose-response effect, with the strongest associations observed for those with moderately elevated levels of CRP and IL-6 as opposed to severely elevated levels. One factor that may have contributed to the lack of dose-response is acute illness in otherwise healthy individuals, which could have resulted in transient elevations in CRP and IL-6. We attempted to control for this by including an indicator for experiencing any of eight common acute health conditions (flu, cold, pneumonia, vomiting, diarrhea, runny nose, cough, or sore throat) in the prior 60 days, but it is possible that some individuals were experiencing transient increases due to other acute conditions not captured in our analysis. It is also important to note that we observed substantial overlap in the confidence intervals for the moderately and severely elevated groups, warranting further investigation into the shape of the dose response curve in future studies.
We also observed that individuals who were overweight (BMI 25-30) or obese (BMI ≥30) had improved thymic function compared to individuals with a BMI <25. Although counterintuitive given that obesity is known to produce elevated levels of CRP and IL-6 (Eder et al. 2009, Visser et al. 1999), which we found were associated with reduced thymic function, these findings are consistent with studies that have identified leptin as a key thymostimulatory agent (Gruver and Sempowski 2008). Leptin is a hormone primarily secreted by adipocytes that contributes to the regulation of energy homeostasis by inhibiting hunger. Individuals who are overweight and obese have elevated levels of and a decreased sensitivity to the leptin hormone (Pan, Guo, and Su 2014, Yang and Barouch 2007), which could provide one explanation for the increased thymic function observed in these groups. Along these lines, it is possible that increased thymic function may not always be health protective and recent animal studies have shown that obesity may result in a cytokine storm in response to immunotherapy, suggesting an overreaction to immune stimulation caused by obesity (Mirsoian et al. 2014). A prior study conducted by Yoshida et al. examined the association between several obesity indicators and thymic function among atomic bomb survivors in Japan (Yoshida et al. 2014). While the authors did not find a statistically significant association between current BMI and thymic function, the authors did observe a statistically significant inverse association between BMI measured 40 years earlier and current thymic function as measured by the number of CD4 sjTRECs. Although this prior study is not directly comparable to ours given the older study population (youngest participant was 58 years of age) and very different distribution of BMI in the population (78% had a BMI less than or equal to 25), it does provide some evidence that early life obesity may actually accelerate thymic involution. Future studies should further tease out the mediating pathways by which obesity impacts thymic function to determine if there may be counteracting effects or sensitive periods across the life course that could be important for modulating health or response to infection.
An important strength of our study is that our data came from a population-representative sample with a high representation of African Americans and individuals with a range of socioeconomic status. We were also able to examine thymic function across a wide age range that included both middle-aged and older adults. Although the present study represents one of the largest studies of thymic function conducted to date, we were nonetheless limited by a relatively small sample size, especially in the youngest age group, which may have reduced precision of effect estimates and limited our ability to detect statistical significance in some instances. In addition, we focused on select measures of physiologic health that have been shown to influence the immune system. Future studies may consider assessing additional biomarkers of the cardiovascular and metabolic systems not assessed in the present study, such as blood pressure, cholesterol, and glycosylated hemoglobin. Assessment of these biomarkers, as well as summary measures of biological risk that incorporate these biomarkers (e.g., allostatic load and Framingham Risk Score), may provide additional insights into the relation of these physiologic systems to thymic function and should be assessed in future studies.
Moreover, it should be noted that the laboratory methods we used to identify naïve T cells (i.e., thymic output) only provide an indirect measurement of thymic function in that the number of sjTREC is proportional but not equivalent to the degree of thymic output of naïve T cells (Douek et al. 1998, Douek et al. 2000, Gruver and Sempowski 2008). However, sjTREC remains one of the most accurate measures of thymic output currently available and quantification of sjTREC has been shown to be robust to sample storage time following cryopreservation (Zubakov et al. 2010). Indeed, sjTREC quantification is a vast improvement over prior methods based on immunophenotyping of CD45RA and co-expression of CD62 ligand, which are uniquely expressed by naïve T cells before cryopreservation but also expressed by memory T cells following cryopreservation (Gruver and Sempowski 2008, Haynes et al. 1999). The more recently described sj/β-TREC ratio, which provides a direct measure of recent thymic emigrants, may overcome the limitations of previous indirect measurements and should be considered as a novel marker of thymic function in future population-based studies (Dion et al. 2004, Ferrando-Martinez, Ruiz-Mateos, and Leal 2010). While the present paper has used the number of sjTRECs as an overall surrogate marker of thymic function, it is important to appreciate that thymic function is not restricted to T cell maturation. The decrease of thymic function as measured by sjTREC levels is strongly tied to thymic involution, hallmarks of which include not only the arrest of T cell development, but also the loss of thymic epithelial cells (TECs) and the substitutions of TECs and true thymic space for adipose tissue (Gruver and Sempowski 2008). Thus, in addition to a reduction in the availability of naïve T cells over the life course, this involution process also affects the production of several peptides that also regulate immunity, as well as other physiologic systems that interact with the thymus in complex feedback loops, such as the thymus-hypothalamus/pituitary axis (Savino 2007, Savino, Arzt, and Dardenne 1999, Savino et al. 1998).
The thymus is a vital organ that is essential to maintaining the naïve T cell pool and mounting an effective immune response. As the immune system is a critical mediator for numerous, if not all health outcomes, understanding the distribution of thymic function at the population level may provide key insights into potential preventative or therapeutic targets to improve population health for a range of health conditions. Indeed, accumulating evidence suggests that the thymus tissue is plastic and elucidating how the process of thymic involution may be reversed with therapeutic interventions is an active area of research (Lynch et al. 2009). While our study supports substantial age-related declines in thymic function in adults, there also appeared to be considerable heterogeneity in thymic function within age categories and by several potential risk factors. In addition, a growing body of literature has documented striking similarities between age-related immunological changes and those associated with exposure to psychosocial stress (Dhabhar 2014) and the thymus in particular has been shown to be sensitive to acute stress-induced atrophy in animal studies (Gruver and Sempowski 2008). Although the thymus is thought to recover following exposure to stressful events, the immune system is left vulnerable during these periods of reduced naïve T cell output, increasing susceptibility to foreign pathogens (Gruver and Sempowski 2008). Whether recurring or prolonged periods of stress-induced atrophy over the life course result in a cumulative health impact in the long-term remains unknown. More research is critically needed to understand the mechanisms that underlie declines in thymic function and why some individuals experience earlier declines than others.
Supplementary Material
Acknowledgements
This research received support from the Population Research Training grant (T32 HD007168) and the Population Research Infrastructure Program (P2C HD050924) awarded to the Carolina Population Center at The University of North Carolina at Chapel Hill by the Eunice Kennedy Shriver National Institute of Child Health and Human Development. The Detroit Neighborhood Health Study, which provided data for this secondary analysis, was funded by the National Institutes of Health (grant numbers: DA22720 and DA022720-S1). Funding for IL-6 and CRP testing was provided by the University of Michigan Nathan Shock Center, Grant AG013283. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
We gratefully acknowledge Dr. Christian Douglas from the Aiello Research Group for her assistance with data management and input on the analytic methods used in this study.
References
- Barrett EL, Richardson DS. Sex differences in telomeres and lifespan. Aging Cell. 2011;10(6):913–21. doi: 10.1111/j.1474-9726.2011.00741.x. doi: 10.1111/j.1474-9726.2011.00741.x. [DOI] [PubMed] [Google Scholar]
- Bauer HM, Ting Y, Greer CE, Chambers JC, Tashiro CJ, Chimera J, Reingold A, Manos MM. Genital human papillomavirus infection in female university students as determined by a PCR-based method. JAMA. 1991;265(4):472–7. [PubMed] [Google Scholar]
- Dhabhar FS. Effects of stress on immune function: the good, the bad, and the beautiful. Immunol Res. 2014;58(2-3):193–210. doi: 10.1007/s12026-014-8517-0. doi: 10.1007/s12026-014-8517-0. [DOI] [PubMed] [Google Scholar]
- Dion ML, Poulin JF, Bordi R, Sylvestre M, Corsini R, Kettaf N, Dalloul A, Boulassel MR, Debre P, Routy JP, Grossman Z, Sekaly RP, Cheynier R. HIV infection rapidly induces and maintains a substantial suppression of thymocyte proliferation. Immunity. 2004;21(6):757–68. doi: 10.1016/j.immuni.2004.10.013. doi: 10.1016/j.immuni.2004.10.013. [DOI] [PubMed] [Google Scholar]
- Douek DC, Koup RA. Evidence for thymic function in the elderly. Vaccine. 2000;18(16):1638–41. doi: 10.1016/s0264-410x(99)00499-5. [DOI] [PubMed] [Google Scholar]
- Douek DC, McFarland RD, Keiser PH, Gage EA, Massey JM, Haynes BF, Polis MA, Haase AT, Feinberg MB, Sullivan JL, Jamieson BD, Zack JA, Picker LJ, Koup RA. Changes in thymic function with age and during the treatment of HIV infection. Nature. 1998;396(6712):690–5. doi: 10.1038/25374. doi: 10.1038/25374. [DOI] [PubMed] [Google Scholar]
- Douek DC, Vescio RA, Betts MR, Brenchley JM, Hill BJ, Zhang L, Berenson JR, Collins RH, Koup RA. Assessment of thymic output in adults after haematopoietic stem-cell transplantation and prediction of T-cell reconstitution. Lancet. 2000;355(9218):1875–81. doi: 10.1016/S0140-6736(00)02293-5. doi: 10.1016/S0140-6736(00)02293-5. [DOI] [PubMed] [Google Scholar]
- Eder K, Baffy N, Falus A, Fulop AK. The major inflammatory mediator interleukin-6 and obesity. Inflamm Res. 2009;58(11):727–36. doi: 10.1007/s00011-009-0060-4. doi: 10.1007/s00011-009-0060-4. [DOI] [PubMed] [Google Scholar]
- Ferrando-Martinez S, Franco JM, Hernandez A, Ordonez A, Gutierrez E, Abad A, Leal M. Thymopoiesis in elderly human is associated with systemic inflammatory status. Age (Dordr) 2009;31(2):87–97. doi: 10.1007/s11357-008-9084-x. doi: 10.1007/s11357-008-9084-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrando-Martinez S, Franco JM, Ruiz-Mateos E, Hernandez A, Ordonez A, Gutierrez E, Leal M. A reliable and simplified sj/beta-TREC ratio quantification method for human thymic output measurement. J Immunol Methods. 2010;352(1-2):111–7. doi: 10.1016/j.jim.2009.11.007. doi: 10.1016/j.jim.2009.11.007. [DOI] [PubMed] [Google Scholar]
- Ferrando-Martinez S, Romero-Sanchez MC, Solana R, Delgado J, de la Rosa R, Munoz-Fernandez MA, Ruiz-Mateos E, Leal M. Thymic function failure and C-reactive protein levels are independent predictors of all-cause mortality in healthy elderly humans. Age (Dordr) 2013;35(1):251–9. doi: 10.1007/s11357-011-9341-2. doi: 10.1007/s11357-011-9341-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrando-Martinez S, Ruiz-Mateos E, Leal M. CD27 and CCR7 expression on naive T cells, are both necessary? Immunol Lett. 2010;127(2):157–8. doi: 10.1016/j.imlet.2009.10.001. doi: 10.1016/j.imlet.2009.10.001. [DOI] [PubMed] [Google Scholar]
- Franco JM, Rubio A, Martinez-Moya M, Leal M, Merchante E, Sanchez-Quijano A, Lissen E. T-cell repopulation and thymic volume in HIV-1-infected adult patients after highly active antiretroviral therapy. Blood. 2002;99(10):3702–6. doi: 10.1182/blood.v99.10.3702. [DOI] [PubMed] [Google Scholar]
- Goldmann E, Aiello A, Uddin M, Delva J, Koenen K, Gant LM, Galea S. Pervasive exposure to violence and posttraumatic stress disorder in a predominantly African American Urban Community: the Detroit Neighborhood Health Study. J Trauma Stress. 2011;24(6):747–51. doi: 10.1002/jts.20705. doi: 10.1002/jts.20705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenland S, Pearl J, Robins JM. Causal diagrams for epidemiologic research. Epidemiology. 1999;10(1):37–48. [PubMed] [Google Scholar]
- Gruver AL, Sempowski GD. Cytokines, leptin, and stress-induced thymic atrophy. J Leukoc Biol. 2008;84(4):915–23. doi: 10.1189/jlb.0108025. doi: 10.1189/jlb.0108025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris JM, Hazenberg MD, Poulin JF, Higuera-Alhino D, Schmidt D, Gotway M, McCune JM. Multiparameter evaluation of human thymic function: interpretations and caveats. Clin Immunol. 2005;115(2):138–46. doi: 10.1016/j.clim.2004.12.008. doi: 10.1016/j.clim.2004.12.008. [DOI] [PubMed] [Google Scholar]
- Haynes BF, Hale LP, Weinhold KJ, Patel DD, Liao HX, Bressler PB, Jones DM, Demarest JF, Gebhard-Mitchell K, Haase AT, Bartlett JA. Analysis of the adult thymus in reconstitution of T lymphocytes in HIV-1 infection. J Clin Invest. 1999;103(6):921. doi: 10.1172/jci5201e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazenberg MD, Borghans JA, de Boer RJ, Miedema F. Thymic output: a bad TREC record. Nat Immunol. 2003;4(2):97–9. doi: 10.1038/ni0203-97. doi: 10.1038/ni0203-97. [DOI] [PubMed] [Google Scholar]
- Horesh D, Lowe SR, Galea S, Uddin M, Koenen KC. Gender Differences in the Long-Term Associations between Posttraumatic Stress Disorder and Depression Symptoms: Findings from the Detroit Neighborhood Health Study. Depress Anxiety. 2014 doi: 10.1002/da.22267. doi: 10.1002/da.22267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izrael D, Battaglia MP, Frankel MR. Proceedings from the 2009 SAS Global Forum. Cary, NC: 2009. Extreme Survey Weight Adjustment as a Component of Sample Balancing (a.k.a. Raking) [Google Scholar]
- Izrael D, Hoaglin DC, Battaglia MP. Proceedings of the Twenty-Fifth Annual SAS Users Group International Conference. Cary, NC: 2000. A SAS Macro for Balancing a Weighted Sample. [Google Scholar]
- Jamieson BD, Douek DC, Killian S, Hultin LE, Scripture-Adams DD, Giorgi JV, Marelli D, Koup RA, Zack JA. Generation of functional thymocytes in the human adult. Immunity. 1999;10(5):569–75. doi: 10.1016/s1074-7613(00)80056-4. [DOI] [PubMed] [Google Scholar]
- Kolte L. Thymic function in HIV-infection. Dan Med J. 2013;60(4):B4622. [PubMed] [Google Scholar]
- Lorenzi AR, Patterson AM, Pratt A, Jefferson M, Chapman CE, Ponchel F, Isaacs JD. Determination of thymic function directly from peripheral blood: a validated modification to an established method. J Immunol Methods. 2008;339(2):185–94. doi: 10.1016/j.jim.2008.09.013. doi: 10.1016/j.jim.2008.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch HE, Goldberg GL, Chidgey A, Van den Brink MR, Boyd R, Sempowski GD. Thymic involution and immune reconstitution. Trends Immunol. 2009;30(7):366–73. doi: 10.1016/j.it.2009.04.003. doi: 10.1016/j.it.2009.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch HE, Sempowski GD. Molecular measurement of T cell receptor excision circles. Methods Mol Biol. 2013;979:147–59. doi: 10.1007/978-1-62703-290-2_12. doi: 10.1007/978-1-62703-290-2_12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MedlinePlus [Internet] National Library of Medicine (US) C-reactive protein, Last Modified. 2013 Feb 11 Accessed June 5. http://www.nlm.nih.gov/medlineplus/ency/article/003356.htm. [Google Scholar]
- Mirsoian A, Bouchlaka MN, Sckisel GD, Chen M, Pai CC, Maverakis E, Spencer RG, Fishbein KW, Siddiqui S, Monjazeb AM, Martin B, Maudsley S, Hesdorffer C, Ferrucci L, Longo DL, Blazar BR, Wiltrout RH, Taub DD, Murphy WJ. Adiposity induces lethal cytokine storm after systemic administration of stimulatory immunotherapy regimens in aged mice. J Exp Med. 2014;211(12):2373–83. doi: 10.1084/jem.20140116. doi: 10.1084/jem.20140116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell WA, Lang PO, Aspinall R. Tracing thymic output in older individuals. Clin Exp Immunol. 2010;161(3):497–503. doi: 10.1111/j.1365-2249.2010.04209.x. doi: 10.1111/j.1365-2249.2010.04209.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer DB. The effect of age on thymic function. Front Immunol. 2013;4:316. doi: 10.3389/fimmu.2013.00316. doi: 10.3389/fimmu.2013.00316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan H, Guo J, Su Z. Advances in understanding the interrelations between leptin resistance and obesity. Physiol Behav. 2014;130:157–69. doi: 10.1016/j.physbeh.2014.04.003. doi: 10.1016/j.physbeh.2014.04.003. [DOI] [PubMed] [Google Scholar]
- Pennell LM, Galligan CL, Fish EN. Sex affects immunity. J Autoimmun. 2012;38(2-3):J282–91. doi: 10.1016/j.jaut.2011.11.013. doi: 10.1016/j.jaut.2011.11.013. [DOI] [PubMed] [Google Scholar]
- Poulin JF, Viswanathan MN, Harris JM, Komanduri KV, Wieder E, Ringuette N, Jenkins M, McCune JM, Sekaly RP. Direct evidence for thymic function in adult humans. J Exp Med. 1999;190(4):479–86. doi: 10.1084/jem.190.4.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savino W. Neuroendocrine control of T cell development in mammals: role of growth hormone in modulating thymocyte migration. Exp Physiol. 2007;92(5):813–7. doi: 10.1113/expphysiol.2007.038422. doi: 10.1113/expphysiol.2007.038422. [DOI] [PubMed] [Google Scholar]
- Savino W, Arzt E, Dardenne M. Immunoneuroendocrine connectivity: the paradigm of the thymus-hypothalamus/pituitary axis. Neuroimmunomodulation. 1999;6(1-2):126–36. doi: 10.1159/000026372. [DOI] [PubMed] [Google Scholar]
- Savino W, Villa-Verde DM, Alves LA, Dardenne M. Neuroendocrine control of the thymus. Ann N Y Acad Sci. 1998;840:470–9. doi: 10.1111/j.1749-6632.1998.tb09585.x. [DOI] [PubMed] [Google Scholar]
- Sempowski GD, Hale LP, Sundy JS, Massey JM, Koup RA, Douek DC, Patel DD, Haynes BF. Leukemia inhibitory factor, oncostatin M, IL-6, and stem cell factor mRNA expression in human thymus increases with age and is associated with thymic atrophy. J Immunol. 2000;164(4):2180–7. doi: 10.4049/jimmunol.164.4.2180. [DOI] [PubMed] [Google Scholar]
- Simanek AM, Cheng C, Yolken R, Uddin M, Galea S, Aiello AE. Herpesviruses, inflammatory markers and incident depression in a longitudinal study of Detroit residents. Psychoneuroendocrinology. 2014;50C:139–148. doi: 10.1016/j.psyneuen.2014.08.002. doi: 10.1016/j.psyneuen.2014.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sipahi L, Uddin M, Hou ZC, Aiello AE, Koenen KC, Galea S, Wildman DE. Ancient evolutionary origins of epigenetic regulation associated with posttraumatic stress disorder. Front Hum Neurosci. 2014;8:284. doi: 10.3389/fnhum.2014.00284. doi: 10.3389/fnhum.2014.00284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin M, Aiello AE, Wildman DE, Koenen KC, Pawelec G, de Los Santos R, Goldmann E, Galea S. Epigenetic and immune function profiles associated with posttraumatic stress disorder. Proc Natl Acad Sci U S A. 2010;107(20):9470–5. doi: 10.1073/pnas.0910794107. doi: 10.1073/pnas.0910794107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visser M, Bouter LM, McQuillan GM, Wener MH, Harris TB. Elevated C-reactive protein levels in overweight and obese adults. JAMA. 1999;282(22):2131–5. doi: 10.1001/jama.282.22.2131. [DOI] [PubMed] [Google Scholar]
- Yan J, Greer JM, Hull R, O'Sullivan JD, Henderson RD, Read SJ, McCombe PA. The effect of ageing on human lymphocyte subsets: comparison of males and females. Immun Ageing. 2010;7:4. doi: 10.1186/1742-4933-7-4. doi: 10.1186/1742-4933-7-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang R, Barouch LA. Leptin signaling and obesity: cardiovascular consequences. Circ Res. 2007;101(6):545–59. doi: 10.1161/CIRCRESAHA.107.156596. doi: 10.1161/CIRCRESAHA.107.156596. [DOI] [PubMed] [Google Scholar]
- Ye P, Kirschner DE, Kourtis AP. The thymus during HIV disease: role in pathogenesis and in immune recovery. Curr HIV Res. 2004;2(2):177–83. doi: 10.2174/1570162043484898. [DOI] [PubMed] [Google Scholar]
- Yoshida K, Nakashima E, Kubo Y, Yamaoka M, Kajimura J, Kyoizumi S, Hayashi T, Ohishi W, Kusunoki Y. Inverse associations between obesity indicators and thymic T-cell production levels in aging atomic-bomb survivors. PLoS One. 2014;9(3):e91985. doi: 10.1371/journal.pone.0091985. doi: 10.1371/journal.pone.0091985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zubakov D, Liu F, van Zelm MC, Vermeulen J, Oostra BA, van Duijn CM, Driessen GJ, van Dongen JJ, Kayser M, Langerak AW. Estimating human age from T-cell DNA rearrangements. Curr Biol. 2010;20(22):R970–1. doi: 10.1016/j.cub.2010.10.022. doi: 10.1016/j.cub.2010.10.022. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.