Figure 1.
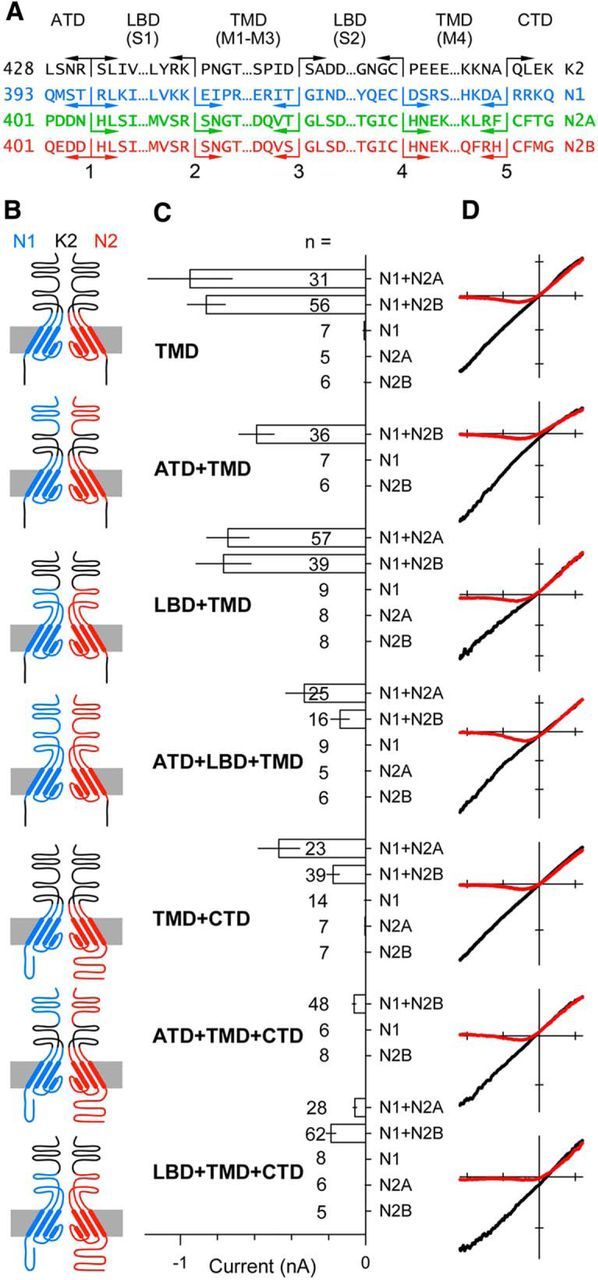
Heteromeric subunit chimeras form functional NMDA receptor pores. A, Protein sequence at the five joints used to construct the chimeric subunits in B. B, Simplified diagram of the four domains in two chimeric subunits of a heteromeric receptor highlighting the pore loop and α helical segments in the TMD derived from the GluN1 (blue) or GluN2 (red) subunit. Non-TMD portions of each chimera derived from the GluK2 kainate receptor subunit are shown in black. Subunits with N1 and N2 extracellular domains are presumed to be adjacent in functional tetramers. For clarity, the subunits are positioned opposite each other in the diagram. C, Mean peak whole-cell agonist-evoked current (± SEM) recorded at −80 mV in HEK 293 cells transiently transfected with chimeric subunit cDNAs and activated with either 10 μm kainate (LBD from GluK2) or 10 μm NMDA and 10 μm glycine (LBDs from NMDA receptor subunits). Agonist-evoked inward currents were significant (i.e., different from zero, t-statistic) for all of the coexpressed construct combinations, but not for any of the chimeric constructs expressed alone. D, I–V for agonist-evoked current recorded as the membrane potential was ramped from −110 to +60 mV at 0.75 mV ms −1 in the presence (red) or absence (black) of 1 mm external magnesium. All recordings from GluN1/2B chimeras. Voltage axis ticks, 50 mV; current axis ticks, 200 pA; except TMD, 500 pA.
