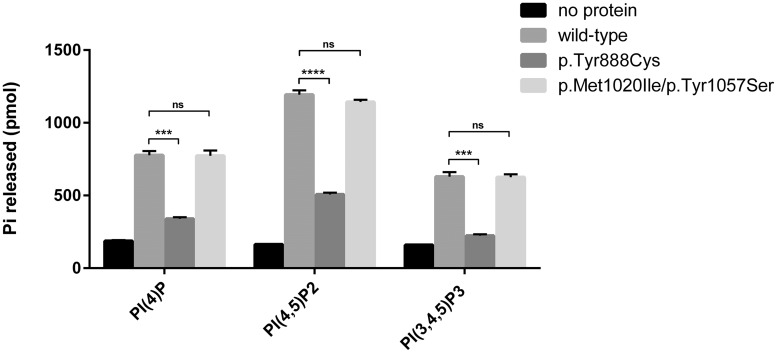
Figure 2.
Phosphatase activity assay of the identified SYNJ1 missense variants. The effect on enzymatic activity of SYNJ1 was tested for mutants resulting from missense variants. Wild-type or mutant FLAG-tagged SYNJ1 (NM_203446.2) was expressed in Expi293F cells, purified and tested for enzymatic activity against short chain phosphoinositides using a malachite-based assay (Maehama et al., 2000). The dephosphorylation of domain specific substrates was investigated: PI(4)P for the Sac1 domain, and PI(4,5)P2 and PI(3,4,5)P3 for the 5’PP domain. Because dephosphorylation of PI(4,5)P2 by the 5’PP domain results in PI4P, which can then be dephosphorylated by the Sac1 domain, the phosphate released when using PI(4,5)P2 as substrate reflects the action of both phosphatase domains. When PI(3,4,5)P3 was used as a substrate, selective action of the 5’PP domain could be assessed. Graphics depict mean values of released inorganic free phosphate from three independent experiments (bars reflect standard error of mean). A clear reduction, but not a complete loss, of phosphate activity towards all tested substrates was found for the p.Tyr888Cys substitution identified in Family A. Compared to wild-type expressing cells, no differences in enzymatic activity was found for cells expressing the p.Met1020Ile/p.Tyr1057Ser substitutions. Unpaired t-test was used to analyse the difference between wild-type and mutants (***P = 0.0002–0.0003, ****P < 0.0001). ns = non-significant.