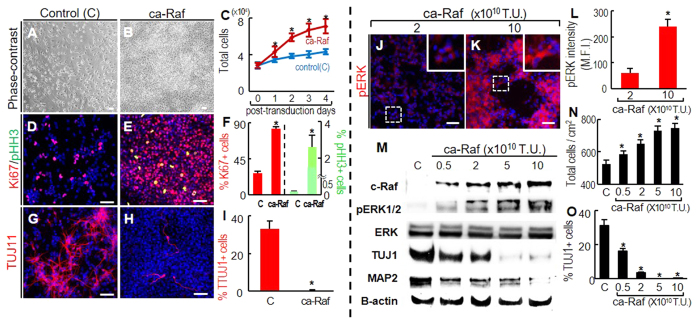
Figure 1. Raf-Erk signal activation at high NSC densities induces cell proliferation and inhibition of neuronal differentiation.
(A–I), To obtain cultures at 50–80% cell confluence, NSCs from E14 rat embryonic cortices were plated at 3–4 × 104/cm2 in FGF2-supplemented N2 medium, and transduced with ca-Raf or control virus (viral titers: 10 × 1010 transduction unit, TU) on the following day. The cells were further cultured in N2 (without FGF2) for 4 days. Cell numbers on post-transduction days 0–4 (A–C), and numbers of cells positive for the proliferation markers Ki67 and M-phase pHH3 (D–F), and for the neuronal marker TUJ1 (G–I) were counted on post-transduction day 4. *p < 0.001, n = 3, Student’s t-test. Scale bar = 50 μm. (J–O), Dose-dependent ca-Raf effects on proliferation and neuronal differentiation. Levels of Erk activation were readily controlled by varying ca-Raf viral titers (J–M). Cortical NSC cultures (plated at 4 × 104/cm2) were transduced with 0.5–10 × 1010 TU of ca-Raf virus, and stained with fluorescent pERK1/2 antibody 2 days later (J–L). Insets, high-magnification images of the boxed areas. Scale bar = 50 μm. Erk activation in individual cells was estimated from the mean fluorescence intensity (MFI) of individual pERK1/2 -stained cells using LAS image analysis (Leica). 54(2 × 1010 TU) and 63(10 × 1010 TU) stained cells were selected at random, and the margins of the individual cells were drawn. MFIs within the outlined area were calculated by comparison with the overall background intensity (L). *p < 0.001, Student’s t-test. Erk activation (pERK1/2 protein level) in cultures transduced with different titers of ca-Raf virus was further analyzed by western blotting (M). Dose-dependent Raf-Erk effects on cell proliferation and neuronal differentiation were estimated by counting total viable cells (N) and neurons positive for TUJ1 (O) in cultures 6 days after transduction. *p < 0.001, n = 3 cultures, one-way ANOVA.