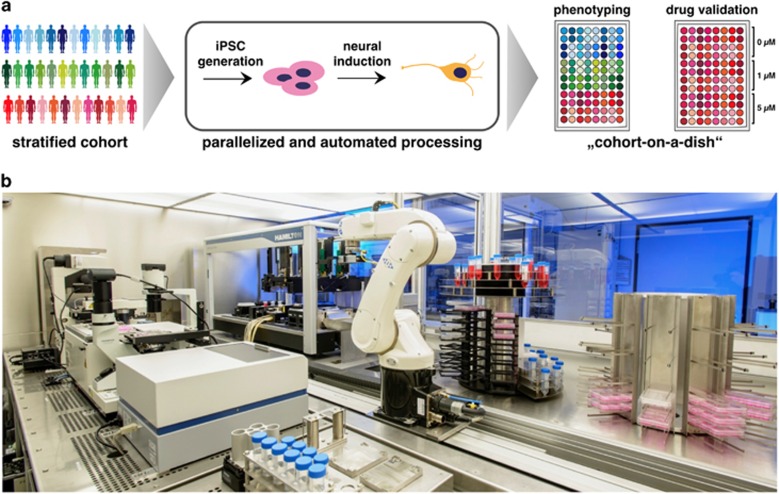
Figure 2.
Automated production and differentiation of iPSCs. (a) Conventional disease modeling or drug evaluation approaches mostly rely on a small number of disease-specific, as well as control iPSC lines and largely ignore the impact of genetic variability on pathological pathways or drug targets. Parallelization of reprogramming and subsequent differentiation would allow assessing phenotypic variation or to validate candidate drugs on multiple genomic backgrounds, for example, stratified patient or control cohorts. (b) Fully integrated robotic systems such as the StemCellFactory (www.stemcellfactory.de) are expected to allow high-throughput reprogramming and differentiation under controlled and standardized conditions, and thus to minimize line-to-line heterogeneity induced by non-standardized manual handling steps. Kindly provided by Andreas Elanzew, Simone Haupt (Life & Brain, Bonn, Germany) and the Fraunhofer Institute for Production Technology (IPT). iPSC, induced pluripotent stem cell.