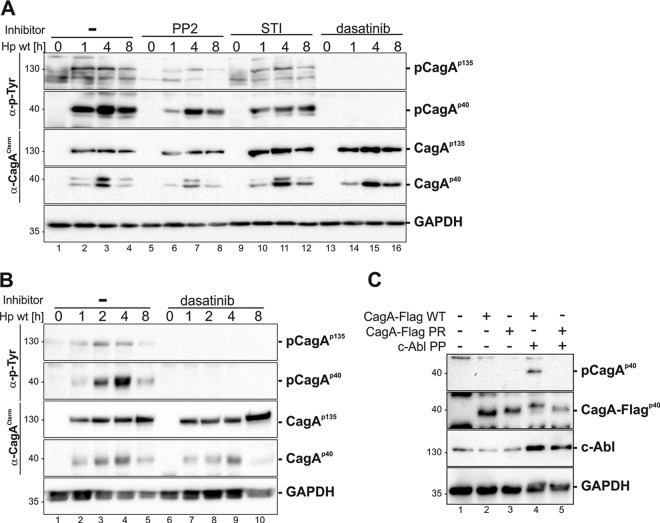
FIG 4.
Tyrosine phosphorylation of H. pylori CagA by coordinated Src and c-Abl activities. (A) MEC1 cells were treated with 10 μM PP2, 10 μM STI-571, and 10 μM dasatinib prior to infection or remained untreated (−). (B) U937 cells were pretreated with 10 μM dasatinib or remained untreated (−). Cells were infected with an H. pylori (Hp) wild-type strain for the indicated time periods. Whole-cell lysates were analyzed by immunoblotting using an anti-phospho-tyrosine (α-p-Tyr) antibody to detect phosphorylated full-length CagA (p-CagAp135) and a C-terminal CagA fragment (p-CagAp40). A monoclonal anti-CagA antibody recognizing the C-terminal part of CagA (α-CagACterm) was applied to verify full-length and fragmented CagA (CagAp135 and CagAp40). As a loading control, the blot was reprobed with an anti-GAPDH antibody. (C) MEC1 cells were cotransfected with cDNAs encoding CagA-Flag wild-type (WT) or phospho-resistant CagA (CagA-Flag PR) with a plasmid expressing constitutively active c-Abl (c-Abl PP), as indicated. Protein lysates were analyzed for the phosphorylated C-terminal CagA fragment (p-CagAp40). To detect the C-terminally Flag-tagged CagA (CagAp40-Flag), an anti-Flag antibody was used. c-Abl and GAPDH were applied as controls.