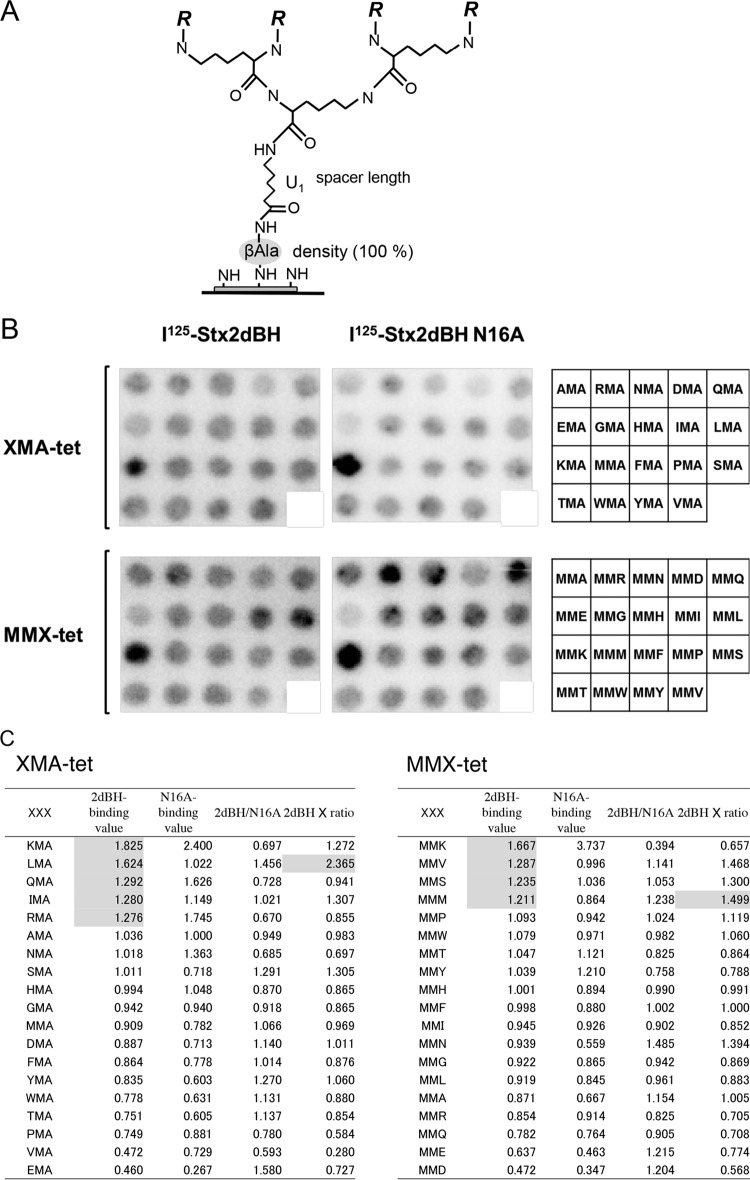
FIG 1.
Identification of Asn16-dependent 2dBH binding motifs by screening of tetravalent peptides synthesized on a cellulose membrane. (A) General structure of the tetravalent peptides synthesized on a cellulose membrane as described previously (37). The density of the tetravalent peptide was maximized by using Fmoc-β-Ala-OH without butoxycarbonyl-β-Ala-OH for the first peptide synthesis cycle. After the addition of one aminohexanoic acid (U) as a spacer following the first β-Ala, Fmoc-Lys(Fmoc)-OH was used for the next two cycles to form four branches in the peptide chain for subsequent synthesis of the various motifs examined in this study (R = Met-Ala-[indicated motif]-Ala-). (B) The tetravalent form of the XMA-RRRR or MMX-RRRR (X indicates any amino acid except Cys) motif was synthesized on a membrane (left and center). The first three amino acids present in the motif are indicated on the right. The membrane was blotted with 125I-2dBH or 125I-2dBH-N16A (1 μg/ml), and the radioactivity bound to each peptide spot was quantified as a pixel value. (C) The sum of the pixel values of all of the peptide spots was normalized to 19 (the number of tetravalent peptides synthesized on the membrane) so that each peptide would have a value of 1 in the absence of selectivity. The ratio of 125I-2dBH binding (2dBH-binding value) to 125I-2dBH-N16A binding (N16A-binding value) (2dBH/N16A) was calculated, and the sum of each ratio was also normalized to 19 to evaluate the specificity of binding through Asn16. The product of the 2dBH-binding value and the normalized 2dBH/N16A ratio (2dBH × ratio) was used to evaluate both binding intensity and specificity. The sequences were sorted in descending order on the basis of the 2dBH-binding values. 2dBH-binding values of >1.20 and the highest 2dBH × ratio products are shaded.