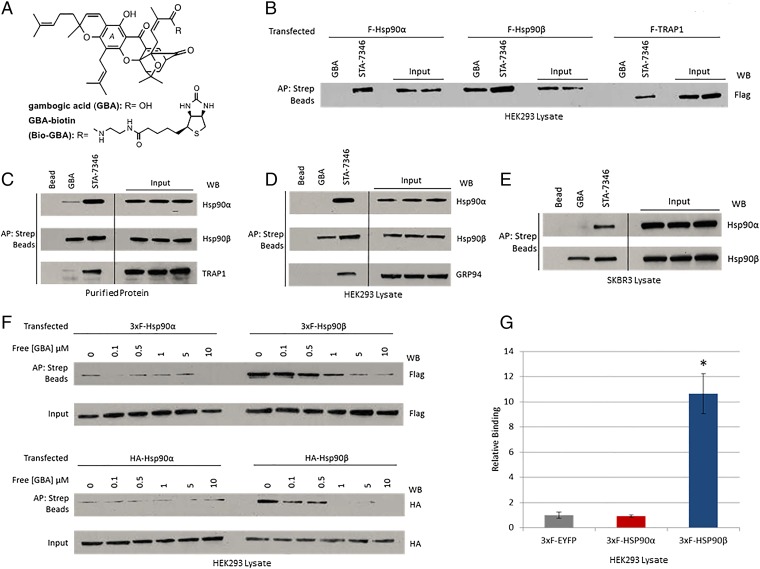
Fig. 1.
Gambogic acid preferentially binds to Hsp90β. (A) Chemical structures of gambogic acid and GBA-biotin. (B–E) Hsp90 from cell lysate or purified protein was isolated with biotinylated GBA and streptavidin beads (abbreviated as Strep beads in all figures). STA-7346, the biotinylated version of the NTD-targeted inhibitor ganetespib, was used for comparison. AP, affinity purification; WB, Western blot. (B) FLAG-Hsp90α, FLAG-Hsp90β, and FLAG-TRAP1 were transfected into HEK293 cells. Only Hsp90β bound to Bio-GBA, whereas STA-7346 interacted strongly with all three isoforms. (C) Purified proteins (10 μg; Hsp90α, Hsp90β, and TRAP1) were incubated with Bio-GBA and STA-7346. Bio-GBA bound strongly to Hsp90β, only weakly to Hsp90α, and not at all to TRAP1. (D and E) Binding preferences of endogenous Hsp90α, Hsp90β, and GRP94 were evaluated in lysate from HEK293 (D) and SKBR3 (E) cells. Bio-GBA bound only to Hsp90β, whereas STA-7346 bound to all isoforms tested. (F) HEK293 cells were transfected with 3×F(LAG)-Hsp90α, 3×F-Hsp90β, HA-Hsp90α, or HA-Hsp90β. The following day, cells were lysed and lysates were treated with increasing concentrations of unlabeled GBA. After incubation for 30 min, Hsp90 protein was isolated with Bio-GBA and streptavidin beads. Compared with 3×F-Hsp90α and HA-Hsp90α, 3×F-Hsp90β and HA-Hsp90β bound strongly to Bio-GBA and binding was competitively and dose-dependently inhibited by pretreatment of cell lysate with unlabeled GBA. (G) LUMIER analysis confirmed that Bio-GBA binds to 3×F-Hsp90β at 10 times the binding strength of 3×F-Hsp90α [using lysates of HEK293 cells transfected with Hsp90 and control (EYFP) vector expression plasmids]. Error bars represent SDs. *P < 0.05.