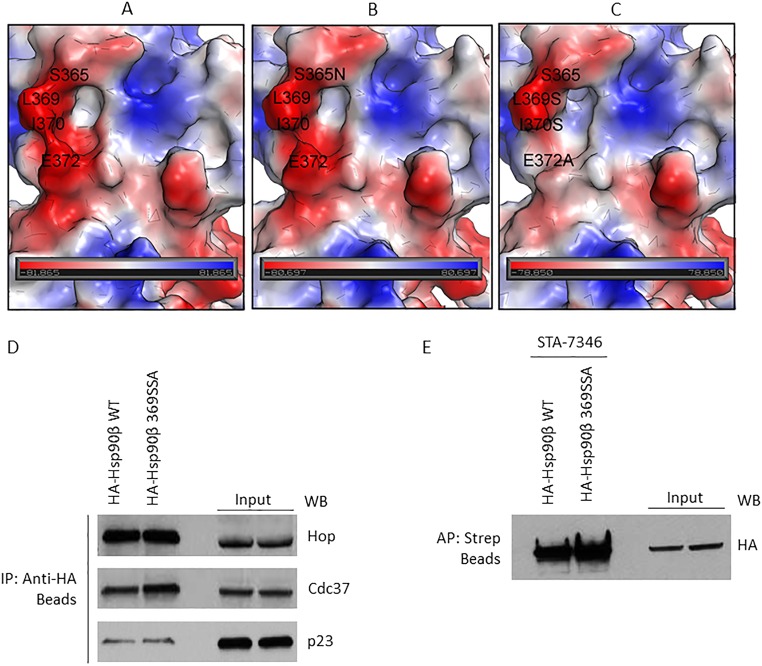
Fig. S3.
Surface electrostatic potential map of the GBA-binding site (residues 350 to 436) in Hsp90β and the impact of binding-site mutation on the interaction of cochaperones. (A) Wild-type human Hsp90β. (B) S375N mutant. (C) Triple mutant L369S, I370S, E372A. Red color corresponds to negatively charged areas, blue color corresponds to positively charged areas, and white color corresponds to neutral areas. The negatively charged area around L369 is because of the D367 (not shown). (D) Plasmids expressing HA-Hsp90β or HA-Hsp90β 369SSA (triple mutant) were transfected into HEK293 cells and then isolated with anti-HA beads. (E) HA-Hsp90β and HA-Hsp90β 369SSA were transfected into HEK293 cells (4 μg plasmid per 10-cm dish) and allowed to express overnight. After 24 h, transfected proteins were immunoprecipitated with HA beads (D) or affinity-purified with STA-7346 and streptavidin beads (E). In D, three cochaperones associated to an equivalent degree with wild-type and mutated Hsp90β. In E, the biotinylated N-terminal inhibitor STA-7346 affinity-purified both wild-type and mutant Hsp90β with equal efficiency. Taken together, the data in D and E confirm that Hsp90β 369SSA is structurally intact.