Abstract
Sigma-1 receptors (σ-1R) are interorganelle signaling molecules, which have been implicated in synaptic plasticity, primarily by enhancing the function of N-methyl-d-aspartate receptors (NMDARs). On the other hand, excessive influx of calcium via activated NMDAR can cause excitotoxicity. Yet, despite their NMDAR-enhancing role, multiple lines of evidence suggest that σ-1Rs are involved in neuroprotection. The mechanism underlying these intriguing opposing effects is not known. Recent studies now suggest the possibility that σ-1Rs could exert neuroprotective effects via targeted disruption of protein-protein interactions between NMDARs and their associated intracellular signaling machinery, specifically the neuronal nitric oxide synthase (nNOS). This targeted disruption of protein-protein interactions between NMDARs and nNOS results in lower levels of nitric oxide generation, thus having a neuroprotective effect. Here, we briefly summarize aspects of σ-1R-mediated enhancement of NMDAR function and possible neuroprotection. In-depth mechanistic understanding of σ-1R modulation of NMDAR function, which preserves Ca2+ homoeostasis while limiting excitotoxicity would provide valuable information for designing novel as well as improving prevailing therapeutic strategies.
Key Words: Sigma-1 receptor, NMDA receptors, Schizophrenia, Neuroprotection
A remarkable number of intra- and extracellular components in a highly organized and intricate manner mediate neurons’ health and spatiotemporal communication. Among many components, calcium ion (Ca2+) has a prominent role and, in fact, stands at the crossroads of having either beneficial (e.g., synaptic plasticity) or toxic (e.g., excitotoxicity) effects on the neuron [1,2,3]. An optimal and regulated intracellular influx of Ca2+ into the neuron elicits signaling cascades that strengthen the communication between synapses (i.e., underlying synaptic plasticity) [3]. However, an excess influx of Ca2+ can also elicit signaling cascades, which in this case may result in a toxic insult to neurons and glia [1]. While the overarching mechanisms underlying the dichotomous effects of Ca2+ are not fully understood, several seminal discoveries have demonstrated a predominant role for N-methyl-d-aspartate receptors (NMDARs), more specifically the NMDAR-associated intracellular signaling machinery, in mediating the dichotomous effects of Ca2+ [4,5]. Not surprisingly, neurons employ a number of specialized mechanisms to govern and tailor the function of NMDARs and/or the NMDAR-associated intracellular signaling machinery. These include mainly, but are not limited to, engaging modulators. The sigma-1 receptor (σ-1R) is one such modulator, which is known to enhance the function of NMDARs (i.e., heightened Ca2+ influx via NMDARs); however, σ-1Rs can also prevent the Ca2+-induced toxicity by modulating the function of a specific NMDAR-associated intracellular signaling component (neuronal nitric oxide synthase, nNOS), which generates toxic species (nitric oxide, NO). These intriguing opposing effects are discussed here. We provide a brief overview on NMDARs and σ-1Rs and then summarize mechanistic aspects of σ-1R modulatory action on the function of NMDARs and its association with nNOS.
NMDARs are glutamate-gated Ca2+-permeable ion channels [6]. They are heterotetrameric assemblies of two compulsory GluN1 subunits together with either two GluN2 (A-D) subunits or a combination of GluN2 or GluN3 (A and B) subunits. These glutamatergic receptors display a characteristic subunit- and age-dependent temporal and spatial distribution throughout the central nervous system. At the synapse, NMDARs exist as large macromolecular complexes that contain numerous types of molecules, including scaffold/signaling proteins, e.g., postsynaptic density-95 (PSD-95). The Ca2+ conductance through NMDARs at synapses acts as a regulator of excitatory synaptic transmission, and any sort of deregulation in NMDAR function leads to neurological disorders, including schizophrenia and stroke [7].
σ-1Rs are intracellular proteins which primarily reside on membranes of the endoplasmic reticulum that are in juxtaposition to mitochondria [8]. They are also present on the plasma membrane and are ubiquitous in neuronal and non-neuronal cells. It is predicted that σ-1Rs possess two transmembrane units along with short N- and long C-terminus tails. Recent investigations have disclosed σ-1R as an interorganelle ‘modulator’ that has chaperone-like activity and also acts as an intracellular sensor in regulating Ca2+ homoeostasis [8,9]. Furthermore, σ-1Rs have been implicated in several physiological functions, including shaping neuronal excitability and long-term potentiation, mainly through functional modulation of ion channels (e.g., NMDARs) [10].
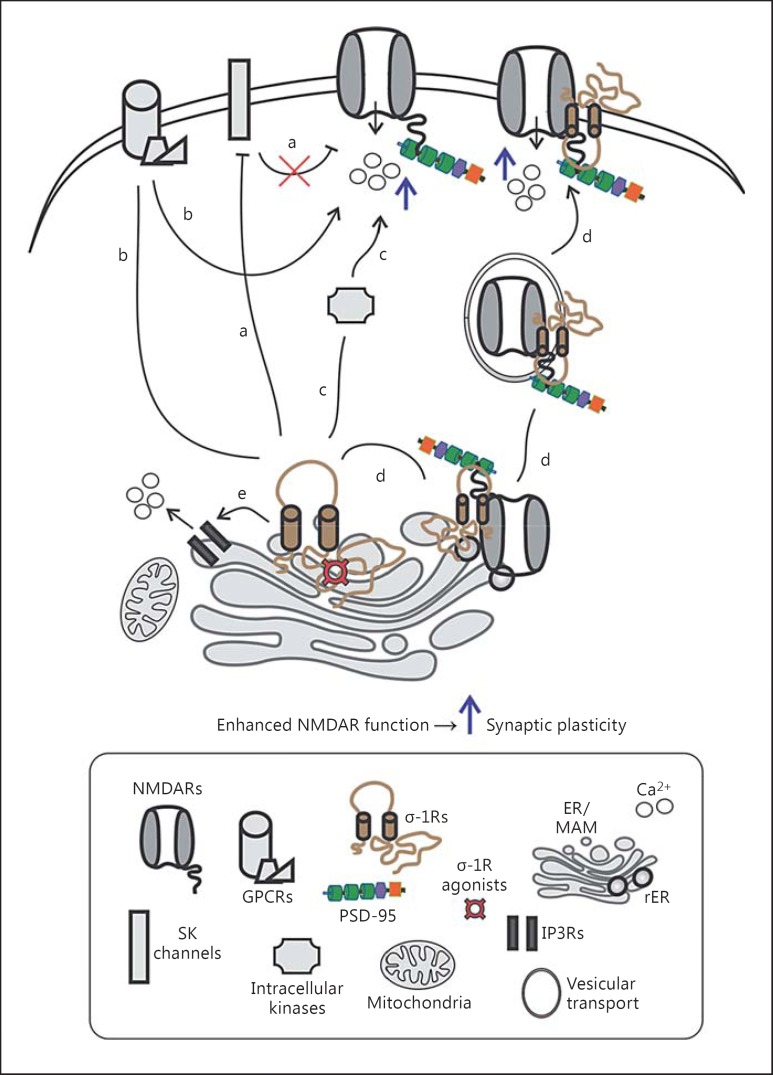
Electrophysiology-based investigations demonstrated that activated σ-1R enhances the frequency and amplitude of various NMDAR-mediated responses as well as NMDAR-dependent long-term potentiation [11,12,13]. Additionally, concurrent behaviour-based studies demonstrated improvements in learning and memory behaviour of animals after the activation of σ-1Rs that are experiencing NMDAR antagonism-induced amnesia [14,15]. Two important features can be inferred from these multimodal studies: first, low doses of σ-1R agonists enhance NMDAR function, while high doses (≥ 10 µM) of σ-1R agonists do not enhance NMDAR function [12,13]. At the moment, it is unclear why high doses of σ-1R agonists do not promote NMDAR function, but it is proposed that at high doses σ-1R agonists can cross-react with NMDARs at their pore sites and eventually block ion channel conductance [16]. Second, the ameliorative effect on NMDAR function by σ-1R agonists can be observed in minutes and sustained for hours [11]. This time scale window reflects the possibility that σ-1Rs may accomplish the functional enhancement of NMDARs through both direct and indirect (i.e., engagement of multiple cellular components) mechanisms (fig. 1). Indeed, σ-1Rs were shown to directly interact with GluN1 subunit of NMDARs [17], and this interaction may explain some of the facilitatory effects of σ-1R agonists on NMDARs. On the other hand, electrophysiology-based examinations have revealed σ-1R-mediated recruitment of small-conductance K+-activated Ca2+ channels (SK channels) [11], G proteins [18], and intracellular kinases (e.g., members of the Src family of kinases [19]) for increasing the function of NMDARs, but with the shortcomings that many aspects remain untested, such as, for instance, the effect of σ-1R agonists on NMDAR subunit-dependent biophysical properties (e.g., gating, single-channel conductance, and open probability). This is especially important in lieu of changes in phosphorylation status - a posttranslational modification that influences the biophysical properties of an ion channel - of NMDAR GluN2B subunits following treatment with σ-1R agonists [19]. Several other reports also demonstrate an increased phosphorylation of GluN1 subunits of NMDAR by kinases such as protein kinases A and C [20,21] after ligand activation of σ-1Rs. Furthermore, a recent biochemistry-based study showed σ-1R-mediated augmentation in expression, trafficking, and surface levels of NMDARs [22]. Equally, another recent report, although through a different viewpoint, demonstrated σ-1R-mediated suppression of NMDAR internalization and obstruction of NMDAR hypofunction [23]. An additional mechanistic modality likely to contribute is the indirect action of σ-1R on NMDAR function through σ-1R-mediated Ca2+ mobilization from the internal stores [e.g., via inositol 1,4,5-triphosphate receptors (IP3Rs) on ER membranes] [9]. A role for σ-1R-mediated Ca2+ mobilization in NMDAR functional augmentation seems plausible, but it remains to be directly tested. Early evidence in support of such a mechanism shows that activation of σ-1Rs in the presence of BAPTA [1, 2-bis(o-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid], a Ca2+ chelating agent, does not induce a functional enhancement of NMDARs [11].
Fig. 1.
σ-1R enhancement of NMDAR function. σ-1Rs adapt a multi-component approach to promote NMDAR function, including the inhibition of SK channels (a), G proteins (identity is unclear; b), and intracellular kinases (c) alongside with an increase in the expression, trafficking, and surface levels (d) of NMDARs. In addition, σ-1R-mediated Ca2+ mobilization from ER via IP3 receptors may contribute to functional enhancement of NMDARs (e). The resultant increase in the influx of Ca2+ (blue arrow) further promotes synaptic plasticity [9,11,18,19,20,21,22].
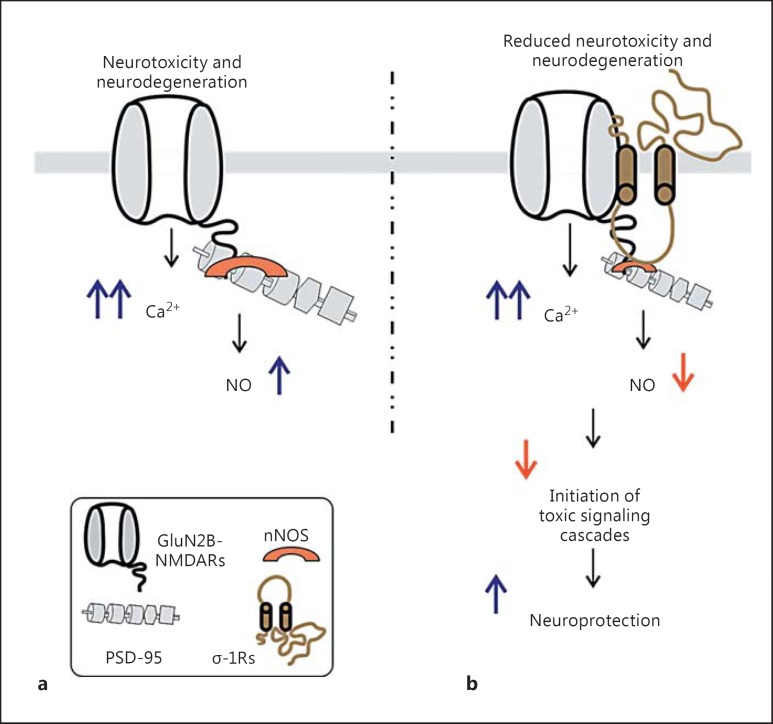
While σ-1R-mediated NMDAR functional enhancement seems semantic and beneficial as it relates to an improvement in the memory behaviour of animals [14,15], it could also provoke an excitotoxic insult to the neuron. However, cell culture and behaviour examinations, based primarily on observations of (a) promotion and attenuation in the protein levels of survival and proapoptotic cellular components [24] and (b) reduction in the production of NO [25], suggest that σ-1R ligands can act as neuroprotective agents. The reduction in the production of NO by σ-1R ligands, if at all in doubt, can be due to the blockade of NMDARs by the ligands. However, this may not be the case. Rather, new findings hint that σ-1Rs may adopt an intriguing approach for wielding neuroprotection [22,26], that is, σ-1R-driven attenuation of targeted protein-protein interactions that mediate neurotoxicity. It has been shown that excessive NMDAR-mediated Ca2+ recruits and activates nNOS that is attached to the NMDAR-PSD-95 complex. This tripartite complex is the driving force for neurotoxicity, and a disruption of these interactions between them greatly reduces toxicity-driven neuronal death [27]. Interestingly, agonist activation of σ-1Rs leads to decreased interactions between the NMDAR subunit (GluN2B) and PSD-95 [22] as well as PSD-95 and nNOS [26] (fig. 2), consequently giving rise to reduced neurotoxicity and neurodegeneration.
Fig. 2.
σ-1R-mediated neuroprotection. a In the absence of σ-1R activation, the increased influx of Ca2+ through GluN2B-containing NMDARs can trigger neurotoxicity and neurodegeneration by increasing the activation of nNOS (eventually NO species) that is attached to PSD-95. b Recent investigations have demonstrated that the activation of σ-1Rs leads to diminution of interactions between GluN2B subunits and PSD-95 as well as PSD-95 and nNOS (represented as a decrease in size). Reducing the interactions between proteins that can generate toxic species may be one of the underlying mechanisms of σ-1R-mediated neuroprotection via reduced neurotoxicity and neurodegeneration [22,25,26,27].
Why are these recent findings important? Neurons constantly have to recalculate and acclimatize to ever-changing circumstances and perturbations. How such adaptation occurs is not well characterized, especially in cases of perturbation in neuronal Ca2+ homoeostasis (i.e., too much or too little NMDAR function and Ca2+ signaling [4]). Numerous investigations suggest a critical role for Ca2+ dyshomoeostasis in the pathogenesis of neuropsychiatric and neurodegenerative disorders [28]. On the other hand, modulation of NDMAR function is beneficial under many circumstances and a current target for therapeutic purposes. Thus, the fact that σ-1R employs a multi-component approach to ensure NMDAR functional enhancement while limiting toxicity-inducible elements may represent a fruitful avenue of further investigation, specifically for gaining further insights into the structural (which domains/sequences of proteins) and time-dependent (acute vs. chronic) aspects of the σ-1R-mediated disruption of targeted protein-protein interactions. Further studies are also needed to determine if σ-1R interaction with NMDAR alone plays a critical role in the σ-1R-mediated increase in NMDAR function. Delving deeper into these alluring aspects - σ-1R-mediated, targeted disruption of interaction between NMDAR and nNOS - using σ-1R knockout mice will validate profiling of σ-1Rs as a neuroprotective target.
In conclusion, obtaining in-depth mechanistic insights into σ-1R modulation of NMDAR function that altogether preserves Ca2+ homoeostasis would provide valuable information for designing novel as well as improving prevailing therapeutic strategies.
References
- 1.Choi DW, Maulucci-Gedde M, Kriegstein AR. Glutamate neurotoxicity in cortical cell culture. J Neurosci. 1987;7:357–368. doi: 10.1523/JNEUROSCI.07-02-00357.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bliss TV, Lomo T. Long-lasting potentiation of synaptic transmission in the dentate area of the anaesthetized rabbit following stimulation of the perforant path. J Physiol. 1973;232:331–356. doi: 10.1113/jphysiol.1973.sp010273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dunwiddie TV, Lynch G. The relationship between extracellular calcium concentrations and the induction of hippocampal long-term potentiation. Brain Res. 1979;169:103–110. doi: 10.1016/0006-8993(79)90377-9. [DOI] [PubMed] [Google Scholar]
- 4.Hardingham GE, Bading H. The Yin and Yang of NMDA receptor signalling. Trends Neurosci. 2003;26:81–89. doi: 10.1016/S0166-2236(02)00040-1. [DOI] [PubMed] [Google Scholar]
- 5.Hardingham GE, Fukunaga Y, Bading H. Extrasynaptic NMDARs oppose synaptic NMDARs by triggering CREB shut-off and cell death pathways. Nat Neurosci. 2002;5:405–414. doi: 10.1038/nn835. [DOI] [PubMed] [Google Scholar]
- 6.Paoletti P, Bellone C, Zhou Q. NMDA receptor subunit diversity: impact on receptor properties, synaptic plasticity and disease. Nat Rev Neurosci. 2013;14:383–400. doi: 10.1038/nrn3504. [DOI] [PubMed] [Google Scholar]
- 7.Lau CG, Zukin RS. NMDA receptor trafficking in synaptic plasticity and neuropsychiatric disorders. Nat Rev Neurosci. 2007;8:413–426. doi: 10.1038/nrn2153. [DOI] [PubMed] [Google Scholar]
- 8.Su TP, et al. The sigma-1 receptor chaperone as an inter-organelle signaling modulator. Trends Pharmacol Sci. 2010;31:557–566. doi: 10.1016/j.tips.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hayashi T, Su TP. Sigma-1 receptor chaperones at the ER-mitochondrion interface regulate Ca2+ signaling and cell survival. Cell. 2007;131:596–610. doi: 10.1016/j.cell.2007.08.036. [DOI] [PubMed] [Google Scholar]
- 10.Kourrich S, et al. The sigma-1 receptor: roles in neuronal plasticity and disease. Trends Neurosci. 2012;35:762–771. doi: 10.1016/j.tins.2012.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martina M, et al. The sigma-1 receptor modulates NMDA receptor synaptic transmission and plasticity via SK channels in rat hippocampus. J Physiol. 2007;578(pt 1):143–157. doi: 10.1113/jphysiol.2006.116178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bergeron R, de Montigny C, Debonnel G. Biphasic effects of sigma ligands on the neuronal response to N-methyl-D-aspartate. Naunyn Schmiedebergs Arch Pharmacol. 1995;351:252–260. doi: 10.1007/BF00233244. [DOI] [PubMed] [Google Scholar]
- 13.Liang X, Wang RY. Biphasic modulatory action of the selective sigma receptor ligand SR 31742A on N-methyl-D-aspartate-induced neuronal responses in the frontal cortex. Brain Res. 1998;807:208–213. doi: 10.1016/s0006-8993(98)00797-5. [DOI] [PubMed] [Google Scholar]
- 14.Maurice T, Privat A. SA4503, a novel cognitive enhancer with sigma1 receptor agonist properties, facilitates NMDA receptor-dependent learning in mice. Eur J Pharmacol. 1997;328:9–18. doi: 10.1016/s0014-2999(97)83020-8. [DOI] [PubMed] [Google Scholar]
- 15.Maurice T, et al. PRE-084, a sigma selective PCP derivative, attenuates MK-801-induced impairment of learning in mice. Pharmacol Biochem Behav. 1994;49:859–869. doi: 10.1016/0091-3057(94)90235-6. [DOI] [PubMed] [Google Scholar]
- 16.Fletcher EJ, et al. Blockade by sigma site ligands of N-methyl-D-aspartate-evoked responses in rat and mouse cultured hippocampal pyramidal neurones. Br J Pharmacol. 1995;116:2791–2800. doi: 10.1111/j.1476-5381.1995.tb15928.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Balasuriya D, Stewart AP, Edwardson JM. The sigma-1 receptor interacts directly with GluN1 but not GluN2A in the GluN1/ GluN2A NMDA receptor. J Neurosci. 2013;33:18219–18224. doi: 10.1523/JNEUROSCI.3360-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bergeron R, de Montigny C, Debonnel G. Potentiation of neuronal NMDA response induced by dehydroepiandrosterone and its suppression by progesterone: effects mediated via sigma receptors. J Neurosci. 1996;16:1193–1202. doi: 10.1523/JNEUROSCI.16-03-01193.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li Z, et al. Dehydroepiandrosterone sulfate prevents ischemia-induced impairment of long-term potentiation in rat hippocampal CA1 by up-regulating tyrosine phosphorylation of NMDA receptor. Neuropharmacology. 2006;51:958–966. doi: 10.1016/j.neuropharm.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 20.Yoon SY, et al. An increase in spinal dehydroepiandrosterone sulfate (DHEAS) enhances NMDA-induced pain via phosphorylation of the NR1 subunit in mice: involvement of the sigma-1 receptor. Neuropharmacology. 2010;59:460–467. doi: 10.1016/j.neuropharm.2010.06.007. [DOI] [PubMed] [Google Scholar]
- 21.Kim HW, et al. Activation of the spinal sigma-1 receptor enhances NMDA-induced pain via PKC- and PKA-dependent phosphorylation of the NR1 subunit in mice. Br J Pharmacol. 2008;154:1125–1134. doi: 10.1038/bjp.2008.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pabba M, et al. NMDA receptors are upregulated and trafficked to the plasma membrane after sigma-1 receptor activation in the rat hippocampus. J Neurosci. 2014;34:11325–11338. doi: 10.1523/JNEUROSCI.0458-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sanchez-Blazquez P, et al. The calcium-sensitive sigma-1 receptor prevents cannabinoids from provoking glutamate NMDA receptor hypofunction: implications in antinociception and psychotic diseases. Int J Neuropsychopharmacol. 2014:1–13. doi: 10.1017/S1461145714000029. [DOI] [PubMed] [Google Scholar]
- 24.Zhang Y, et al. Sigma-1 receptor agonists provide neuroprotection against gp120 via a change in bcl-2 expression in mouse neuronal cultures. Brain Res. 2012;1431:13–22. doi: 10.1016/j.brainres.2011.10.053. [DOI] [PubMed] [Google Scholar]
- 25.Goyagi T, et al. Neuroprotective effect of σ1-receptor ligand 4-phenyl-1-(4-phenylbutyl) piperidine (PPBP) is linked to reduced neuronal nitric oxide production. Stroke. 2001;32:1613–1620. doi: 10.1161/01.str.32.7.1613. [DOI] [PubMed] [Google Scholar]
- 26.Yang ZJ, et al. Sigma receptor ligand 4-phenyl-1-(4-phenylbutyl)-piperidine modulates neuronal nitric oxide synthase/postsynaptic density-95 coupling mechanisms and protects against neonatal ischemic degeneration of striatal neurons. Exp Neurol. 2010;221:166–174. doi: 10.1016/j.expneurol.2009.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aarts M, et al. Treatment of ischemic brain damage by perturbing NMDA receptor-PSD-95 protein interactions. Science. 2002;298:846–850. doi: 10.1126/science.1072873. [DOI] [PubMed] [Google Scholar]
- 28.Nedergaard M, Verkhratsky A. Calcium dyshomeostasis and pathological calcium signalling in neurological diseases. Cell Calcium. 2010;47:101–102. doi: 10.1016/j.ceca.2009.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]