Abstract
Aims Left atrial appendage closure is a non-pharmacological alternative for stroke prevention in high-risk patients with non-valvular atrial fibrillation. The objective of the multicentre EWOLUTION registry was to obtain clinical data on procedural success and complications, and long-term patient outcomes, including bleeding and incidence of stroke/transient ischaemic attack (TIA). Here, we report on the peri-procedural outcomes of up to 30 days.
Methods and results Baseline/implant data are available for 1021 subjects. Subjects in the study were at high risk of stroke (average CHADS 2 score: 2.8 ± 1.3, CHA 2 DS 2 -VASc: 4.5 ± 1.6) and moderate-to-high risk of bleeding (average HAS-BLED score: 2.3 ± 1.2). Almost half of the subjects (45.4%) had a history of TIA, ischaemic stroke, or haemorrhagic stroke; 62% of patients were deemed unsuitable for novel oral anticoagulant by their physician. The device was successfully deployed in 98.5% of patients with no flow or minimal residual flow achieved in 99.3% of implanted patients. Twenty-eight subjects experienced 31 serious adverse events (SAEs) within 1 day of the procedure. The overall 30-day mortality rate was 0.7%. The most common SAE occurring within 30 days of the procedure was major bleeding requiring transfusion. Incidence of SAEs within 30 days was significantly lower for subjects deemed to be ineligible for oral anticoagulation therapy (OAT) compared with those eligible for OAT (6.5 vs. 10.2%, P = 0.042).
Conclusion Left atrial appendage closure with the WATCHMAN device has a high success rate in complete LAAC with low peri-procedural risk, even in a population with a higher risk of stroke and bleeding, and multiple co-morbidities. Improvement in implantation techniques has led to a reduction of peri-procedural complications previously limiting the net clinical benefit of the procedure.
Keywords: Stroke, Left atrial appendage, Atrial fibrillation
Introduction
Despite the introduction of new drug therapies for stroke prevention, many patients and physicians continue to seek alternatives for a variety of reasons, including contraindications, medication side effects, and adherence and quality-of-life concerns. Left atrial appendage closure (LAAC) is a non-pharmacological alternative for stroke prevention in high-risk patients with non-valvular atrial fibrillation (AF) in cases where oral anticoagulation therapy (OAT) is deemed not to be the ideal long-term treatment. It was developed based on the finding that around 90% of thrombi in the left atrium originated in the left atrial appendage in this population. 1 Several multicentre randomized studies found the treatment to be safe, effective, and non-inferior to vitamin K antagonists (VKAs) for stroke prevention, 2 , 3 whereas longer-term follow-up supports a potential for superiority and lower mortality of LAAC. 4
Current international guidelines recommend the option of LAAC for high-risk patients with non-valvular AF who are either contraindicated or unsuitable for long-term OAT, at high bleeding risk, or otherwise prefer an alternative. 5–8 In the specific patient population considered optimal by guidelines, there is actually only limited evidence available as it was not part of the randomized controlled trial (RCT). Questions remain with respect to the proper populations based on stroke and bleeding risk factors, considerations of adherence to OAT, and quality-of-life issues aside. In Germany and Switzerland, the therapy was established as part of standard practice >5 years ago, while FDA approval in the USA was obtained only very recently, and in many other countries reimbursement remains a challenge
More broadly, the generalizability of findings from clinical trials into real-world clinical settings that invariably involve individual physician practice and a more diverse patient population is a key question with any new therapy. The prospective, multicentre EWOLUTION registry was designed to examine this question. 9 In addition, a large prospective study can be helpful in better characterizing the incidence of rare events that may not have been observed, or have only been observed in very small frequencies, in previous smaller studies.
Methods
The study adhered to international rules for scientific studies, the Helsinki principles, with local ethics committee approval in all participating centres. All subjects provided informed consent prior to the procedure. Boston Scientific Corporation provided funding for the study.
Briefly, EWOLUTION was designed as a multicentre, prospective, non-randomized cohort study aiming to include over 1000 patients. Subjects were recruited from the general population of clinical sites at each participating physician's discretion if they were eligible to receive the WATCHMAN device according to the appropriate local and international guidelines, and were of legal age to provide informed consent. Implanting physicians and centres with varying levels of past experience with the device participated in the registry. All implanting physicians underwent a thorough training and certification programme to ensure an appropriate level of expertise in order to minimize patient risk.
Follow-up for subjects was based on each institution's standard practice, generally a clinical visit between 1 and 3 months post-procedure, LAA imaging to assess residual flow around the device, and annual follow-up visits. Reporting on adverse events in this study is based on peri-procedural monitoring until 30 days of follow-up post-procedure. The relatedness of each adverse event to the device/procedure was determined by the participating centre. Events and relevant source documents were additionally reviewed by the Sponsor Medical Safety Group (MSG). The MSG includes physicians with expertise in Electrophysiology and/or Cardiology, as well as other healthcare professionals with the necessary therapeutic and subject matter expertise to evaluate the events. Centres were required to provide additional information in case of disagreement with the investigators' assessment of device-relatedness classification. The MSG could overrule the investigator to upgrade an event to become device-/procedure-related. On the other hand, the MSG could never downgrade the centre classification to classify an event as unrelated to the device/procedure. For all patient deaths, source documents were requested at the sites and reviewed by the MSG.
The objective of the study was to obtain data on procedural success and complications, and long-term patient outcomes, including bleeding and incidence of stroke/transient ischaemic attack (TIA), and the sample size was based on a desire to obtain sufficiently precise estimates of rare adverse events and not on power requirements for a formal hypothesis test. This study summarizes the results of the procedure and 30-day follow-up data on all enrolled subjects. The definitions and reporting requirements for adverse event (AE) and serious AE (SAE) are based on ISO 14155 and the MEDDEV 2.7/3 12/2010 and clearly spelled out in the study protocol. The electronic clinical report form provides fields with anticipated SAEs, and open space to report on any other SAE as deemed appropriate by the investigator. The fields obviously included serious events such as perforation, tamponade, embolism, neurological events, thrombosis, and bleeding. Bleeding was scored according to the BARC criteria, 10 and stroke in accordance to criteria described by Leon et al . 11 All centres were monitored by an outside contract research organization (CRO), and all centres were visited at least once to monitor for completeness of the present 30-day follow-up data. Rates of events are calculated via the Kaplan–Meier method to account for censoring. P -values are based on log-rank tests for time-to-event analysis and Fisher's exact test for binomial proportions. Follow-up is ongoing and will continue through 2 years post-implant.
Results
Enrolment opened in October 2013 and was completed in May 2015. A total of 1025 subjects were scheduled for implant in the study in a total of 47 centres in 13 countries. The inclusion rate ranged from 1 to 86 subjects depending on centre volume and local approval to start participation in the trial. Centres were encouraged to enrol consecutive patients to represent real-life practice and avoid selection bias. Baseline and acute implant data are, respectively, available for 1021 and 1019 subjects. Baseline demographics and risk factors are summarized in Table 1 .
Table 1.
Baseline characteristics
| Characteristic | Summary statistics |
|---|---|
| Not eligible for OAT | 61.8% (627/1014) |
| Age at time of consent (years) | 73 ± 9 |
| Median (range) | 75 (39, 94) |
| Age ≥75 | 50.8% (519/1021) |
| Female gender | 40.1% (409/1021) |
| History of TIA | 10.7% (108/1014) |
| History of ischaemic stroke | 19.7% (200/1014) |
| Congestive heart failure | 34.2% (347/1014) |
| History of hypertension | 81.7% (828/1014) |
| Diabetes | |
| Type I | 1.3% (13/1014) |
| Type II | 28.3% (287/1014) |
| Previous haemorrhagic stroke | 15.0% (152/1014) |
| Vascular disease | 41.8% (423/1013) |
| Abnormal renal function | 15.6% (158/1014) |
| Abnormal liver function | 4.2% (43/1014) |
| History of major bleeding | 31.2% (316/1013) |
| Prior major bleeding or predisposition to bleeding | 38.7% (392/1013) |
| Labile INRs | 17.0% (172/1014) |
| Concomitant use of drugs | 27.8% (282/1014) |
| Alcohol abuse | 4.2% (43/1014) |
| CHADS 2 score | |
| 0 | 2.6% (26/1014) |
| 1 | 13.2% (134/1014) |
| 2 | 27.1% (275/1014) |
| 3 | 25.5% (259/1014) |
| 4 | 22.4% (227/1014) |
| 5 | 7.2% (73/1014) |
| 6 | 2.0% (20/1014) |
| CHA 2 DS 2 -VASc score | |
| 0 | 0.3% (3/1013) |
| 1 | 1.6% (16/1013) |
| 2 | 9.7% (98/1013) |
| 3 | 15.4% (156/1013) |
| 4 | 24.0% (243/1013) |
| 5 | 22.7% (230/1013) |
| 6 | 16.8% (170/1013) |
| 7 | 6.5% (66/1013) |
| 8 | 2.7% (27/1013) |
| 9 | 0.4% (4/1013) |
| HAS-BLED score | |
| 0 | 4.4% (45/1013) |
| 1 | 22.0% (223/1013) |
| 2 | 33.3% (337/1013) |
| 3 | 23.4% (237/1013) |
| 4 | 11.9% (121/1013) |
| 5 | 4.3% (44/1013) |
| 6 | 0.5% (5/1013) |
| 7 | 0.1% (1/1013) |
Values presented are % ( N /total) or mean ± standard deviation, median (minimum, maximum).
OAT, oral anticoagulation therapy; TIA, transient ischaemic attack; INRs, international normalized ratios.
Approximately 60% of subjects were male and the mean age was 73 years. Most patients (81.7%) had a history of hypertension and baseline vascular disease was prevalent (41.8%). Approximately 34% had congestive heart failure; over half (55.9%) of these subjects were NYHA Class II and 31.3% were NYHA Class III. Abnormal renal function was observed in 15.6% of subjects. Type II diabetes was present in 28.3% of subjects. Based on the CHADS 2 and CHA 2 DS 2 -VASc risk scores, subjects in the study were at high risk of stroke with an average CHADS 2 score of 2.8 ± 1.3, and CHA 2 DS 2 -VASc score of 4.5 ± 1.6. Almost half of the subjects had a history of either TIA (10.7%), or ischaemic stroke (19.7%), or haemorrhagic stroke (15.0%), thus constituting a very high-risk population. All subjects had a sufficiently high risk for stroke to warrant the use of OAT. However, 62% of patients were deemed unsuitable for OAT by their physician, based on factors such as co-morbidities, the inability to adhere to OAT, and bleeding history or high bleeding risk. Nearly one-third of all subjects had a history of major bleeding (31.2%) and there was a moderate-to-high risk of bleeding with 40% of subjects having a HAS-BLED score of three or more, and an average HAS-BLED score of 2.3 ± 1.2. At baseline, only 31% of patients were on OAT, 21% were on dual antiplatelet therapy (DAPT), 22% were on single APT, and 27% were not taking any form of anticoagulation. Following implant, anticoagulation was increased in line with WATCHMAN recommendations of use in the first 3–6 months; 27% of patients were on OAT, 59% were on dual APT, and 7% were on single APT, while 6% remained without any type of OAT.
Implant procedure success
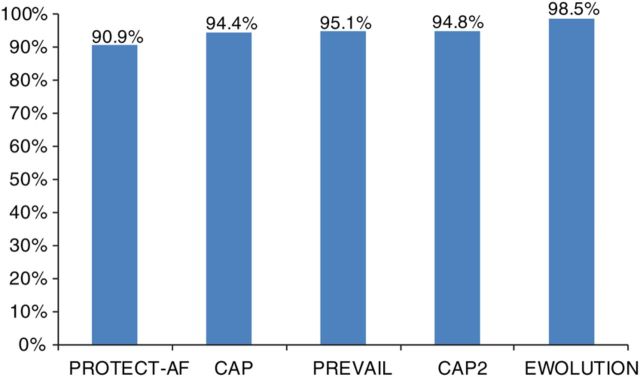
A total of 1019 subjects underwent implant attempts. Five additional subjects were considered to have an unsuitable anatomy for implant after enrolment, while implant status is unknown for one subject. Table 2 summarizes procedural results for the subjects with implant attempts. The device was successfully deployed in 1004 of 1019 subjects (98.5%), comparing favourably with previously reported rates in other WATCHMAN trials 11 ( Figure 1 ). The most common reason for the 15 (1.5%) deployment failures was unfavourable anatomy or mismatch between the size of the device and the LAA. Successful procedural closure of the LAA with no or minimal residual flow (defined as <5 mm assessed via peri-procedural transoesophageal echo, TEE) was achieved in 99.3% of implanted patients with TEE data available (977 of 984 subjects). A total of 1082 devices were used in the context of the study, resulting in an average of only 1.07 devices used per patient. In 92.7% of patients, only one device was used. Most devices were deployed and released directly at the first attempt (71.1%) or recaptured only 1–2 times (22.9%) before successful release.
Table 2.
Procedural results
| Characteristic | All patients |
|---|---|
| Successful deployment | 98.5% (1004/1019) |
| LAA seal | |
| Complete seal | 91.4% (899/984) |
| Jet size ≤5 mm | 7.9% (78/984) |
| Jet size >5 mm | 0.7% (7/984) |
LAA, left atrial appendage.
Figure 1.
Implant success in EWOLUTION when compared with prior WATCHMAN studies.
Implant procedure safety
Procedure through 7 days
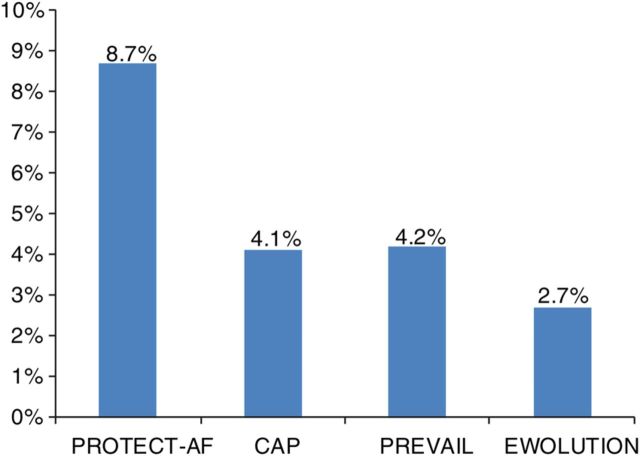
In prior WATCHMAN trials, acute safety was reported up to 7 days post-implant. In the present study, procedure- and/or device-related SAEs within the first 7 days occurred at a rate of 2.8% as summarized in Table 3 . This rate is lower than in any of the prior WATCHMAN LAAC trials as shown in Figure 2 . Twenty-eight subjects experienced 31 SAEs within 1 day of the procedure, of which 25 were deemed by the investigators to be related to the device or the implant procedure. The 31 events included major bleeding ( n = 7), pericardial effusion ( n = 5) including only one cardiac tamponade, vascular damage to the groin ( n = 4), procedural air embolism ( n = 3), device embolization ( n = 2, one retrieved percutaneously and one removed surgically), and reinterventions due to incomplete LAA seal ( n = 2), and several singular events. All but one of these SAEs were managed effectively with complete recovery of the patients. There were three deaths within 7 days of the procedure, all of them reported as not associated with the device or procedure; one due to right ventricular failure the day of the procedure, one due to respiratory insufficiency on Day 4, and one due to cardiac on Day 6.
Table 3.
Kaplan–Meier analysis for serious adverse events by relatedness
| Estimated event rate (%) | 95% CI | |
|---|---|---|
| Any SAE through 1 day | 3.0 | 2.1%, 4.3% |
| Any device-/procedure-related SAE through 1 day | 2.5 | 1.6%, 3.6% |
| Any SAE through 7 days | 4.1 | 3.0%, 5.5% |
| Any device-/procedure-related SAE through 7 days | 2.8 | 1.9%, 4.0% |
| Any SAE through 30 days | 7.9 | 6.3%, 9.8% |
| Any device-/procedure-related SAE through 30 days | 3.6 | 2.5%, 4.9% |
SAE, serious adverse event; CI, confidence interval.
Figure 2.
Serious procedure-/device-related events through 7 days in EWOLUTION when compared with prior WATCHMAN studies.
Procedure through 30 days
There were four additional deaths within 30 days, one of which at Day 19 as a result of an air embolism on the day of the procedure. The other three deaths were reported as unrelated to the procedure; one at Day 10 due to Clostridium difficile infection and two cases of gastrointestinal bleeding at Days 12 and 15. Both subjects were on dual APT at the time of the event. The overall 30-day mortality rate was 0.7%.
Table 4 reports a list of all the 84 SAEs occurring in 73 patients within 30 days. In several patients, more than one SAE, both related and unrelated, was observed. There were 34 SAEs in 32 of 73 patients adjudicated as procedure- or device-related, while the majority of 50 SAEs in 48 of 73 patients were reported as unrelated to the procedure or device. The total 30-day SAE rate was 7.9%, with a reported procedure and/or device-related SAE rate of 3.6%. Major bleeding requiring transfusion (17 patients) was the most common SAE, although only 8 of these were related to groin access (including 3 pseudoaneurysms and 2 laceration of veins), while 4 were due to gastrointestinal bleeding. In these 17 patients, oral anticoagulant (OAC) was used in three, single antiplatelet therapy in five, DAPT in seven, while two used no form of anticoagulation, and HAS-BLED ranged from one to five. There were seven subjects with procedural pericardial effusion, of which three required subxyphoidal ( n = 2) or surgical pericardiocentesis ( n = 1) while four could be managed conservatively, and none had lasting consequences. In three patients, an ischaemic stroke occurred at Days 15, 21, and 23, respectively, none of them resulting in death, and two of three showing complete recovery. Two subjects were on dual APT following implant (CHADS 2 scores were 2 and 3; CHA 2 DS 2 -VASc scores were 3 and 5), while one very high-risk patient was on Clopidogrel alone (CHADS 2 score was 5; CHA 2 DS 2 -VASc score was 8). The latter stroke was reported as an increase in pre-existing hemiparesis and dysarthria and was reported to be procedure-/device-related.
Table 4.
All serious adverse events through 30 days
| Serious adverse events | Device-/procedure-related SAEs ( N = 34) | Percentage of patients experiencing the SAE ( N = 73) | Unrelated SAEs ( N = 50) | Percentage of patients experiencing the SAE ( N = 73) |
|---|---|---|---|---|
| Major bleeding requiring transfusion | 8 | 11.0 | 11 | 15.1 |
| Other bleeding complications (haematoma, haemoptysis, haematuria, and anaemia requiring transfusion) | 2 | 2.7 | 4 | 5.5 |
| Pericardial effusion | 3 | 4.1 | 2 | 2.7 |
| Cardiac tamponade | 2 | 2.7 | 0 | 0.0 |
| Strokes | 1 | 1.4 | 2 | 2.7 |
| Suspected TIA | 0 | 0.0 | 2 | 2.7 |
| Pulmonary embolism | 0 | 0.0 | 1 | 1.4 |
| Air embolism | 3 | 4.1 | 0 | 0.0 |
| Device embolization | 2 | 2.7 | 0 | 0.0 |
| Adverse reaction to anaesthesia | 2 | 1.4 | 0 | 0.0 |
| Reintervention due to incomplete seal | 2 | 2.7 | 0 | 0.0 |
| Vascular damage at puncture site | 5 | 5.5 | 0 | 0.0 |
| Hypotension | 1 | 1.4 | 0 | 0.0 |
| Other cardiovascular conditions | 1 | 1.4 | 12 | 16.4 |
| Heart failure | 0 | 6 | ||
| Mitral regurgitation | 0 | 2 | ||
| Peripheral vascular disease | 0 | 2 | ||
| Chest pain | 1 | 0 | ||
| Asystole | 0 | 1 | ||
| Sick sinus syndrome | 0 | 1 | ||
| Other non-cardiac conditions | 2 | 2.7 | 16 | 19.2 |
| Pulmonary | 1 | 6 | ||
| Systemic infection | 1 | 2 | ||
| Genitourinary | 0 | 2 | ||
| Physical trauma | 0 | 1 | ||
| Cancer | 0 | 1 | ||
| Anaphylactic shock | 0 | 1 | ||
| HEENT | 0 | 1 | ||
| Gastrointestinal | 0 | 1 | ||
| Abnormal lab values | 0 | 1 | ||
| 34 | 43.8 | 50 | 65.8 |
N SAEs = 84 in 73 unique patients. Percentages may add to >100% due to patients appearing in more than one category.
SAE, serious adverse event; TIA, transient ischaemic attack.
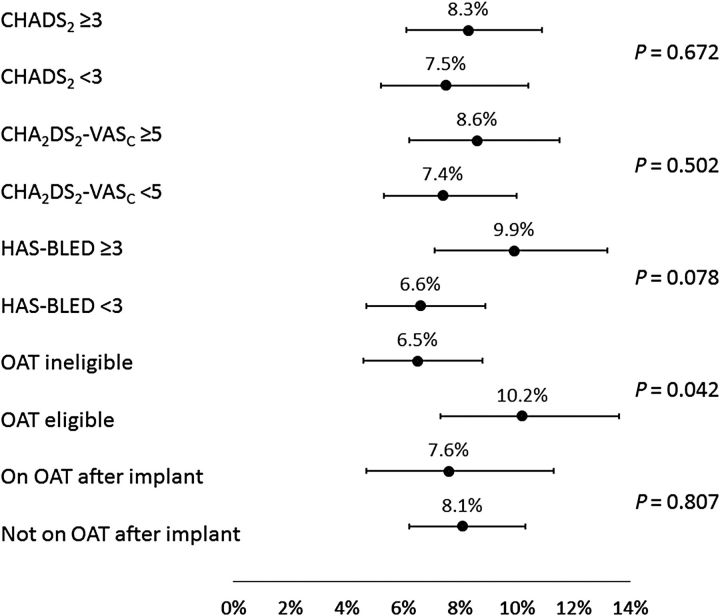
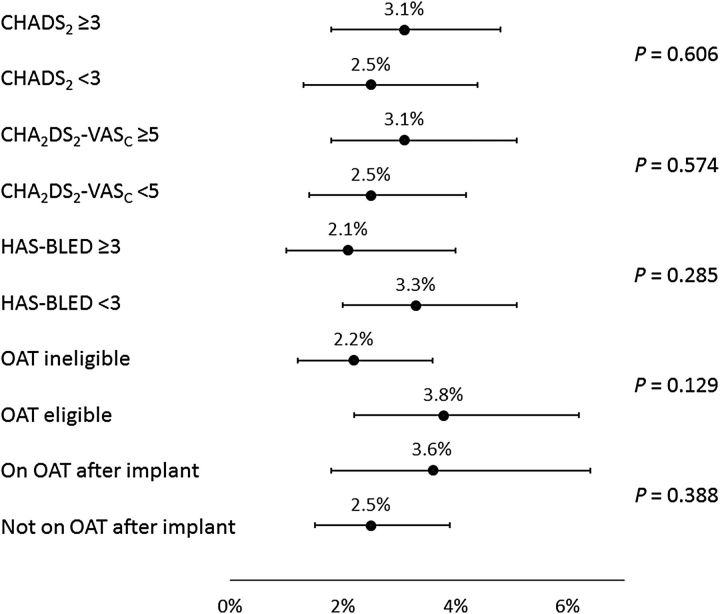
Subgroup analyses
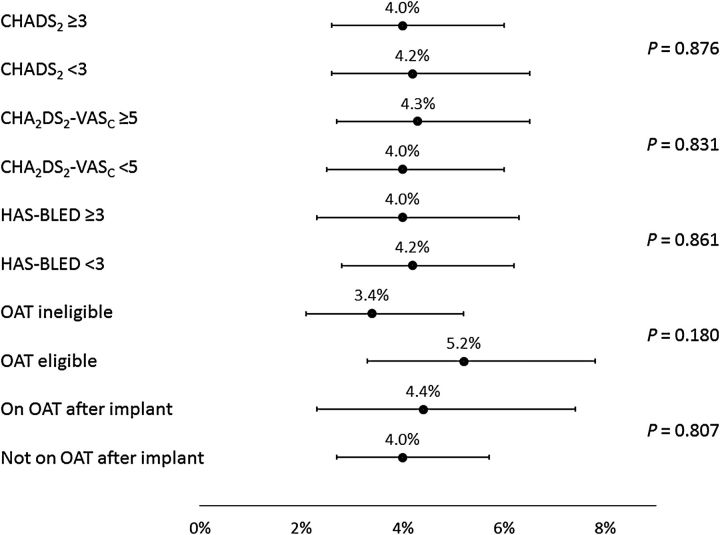
Analyses were performed to examine the consistency of results across subgroups defined by baseline characteristics ( Figures 3–6 ); these included the pre-specified subgroups and cut-off values defined by CHADS 2 , CHA 2 DS 2 -VASc, and HAS-BLED scores, as well as the post hoc subgroups of patients defined by baseline OAT eligibility and whether or not subjects were prescribed OAT at the time of the procedure. Implant success was similar whether or not patients were eligible for OAT (98.4 and 98.7% for those eligible and not eligible, respectively, P = 0.794). The incidence of SAEs through 7 or 30 days of the procedure (whether or not related to the procedure) did not appear to be related to CHADS 2 or CHA 2 DS 2 -VASc scores, nor was it generally different for subjects on OAT after implant vs. subjects not on OAT after implant (all P > 0.39).
Figure 3.
Subgroup results—serious adverse events through 7 days. The percentage (95% confidence interval) of the number of subjects experiencing events is displayed along the horizontal axis, separately for each subgroup defined by baseline characteristics. P -values are based on log-rank tests. OAT, oral anticoagulation therapy.
Figure 6.
Subgroup results for procedure-/device-related serious adverse events through 30 days. The percentage (95% confidence interval) of the number of subjects experiencing events is displayed along the horizontal axis, separately for each subgroup defined by baseline characteristics. P -values are based on log-rank tests. OAT, oral anticoagulation therapy.
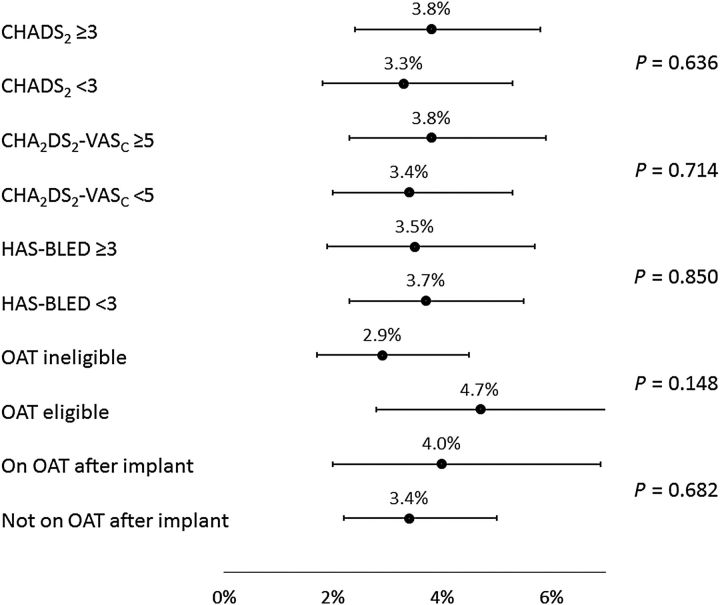
Figure 5.
Subgroup results for serious adverse events through 30 days. The percentage (95% confidence interval) of the number of subjects experiencing events is displayed along the horizontal axis, separately for each subgroup defined by baseline characteristics. P -values are based on log-rank tests. OAT, oral anticoagulation therapy.
However, for those with HAS-BLED <3 vs. ≥3, incidence of SAEs through 30 days showed a trend towards higher event rates with a higher risk (6.6 vs. 9.9%, respectively, P = 0.078). Bleeding was also found to occur more often in patients with a HAS-BLED score of ≥3 (1.7 vs. 4.0%, P = 0.029). For the ischaemic stroke, the event rate was simply too low to find ay statistical relevance. Of note, the incidence of SAEs through 30 days was significantly lower for subjects deemed to be ineligible for OAT compared with subjects eligible for OAT (6.5 vs. 10.2%, P = 0.042). In agreement with previous studies, EWOLUTION also suggests a relationship between implant numbers at centres and parameters such as implant success, complete LAA seal, and number of device recaptures. However, no significant correlation was found between implant numbers or consecutive enrolment adherence at centres and peri-procedural SAEs ( P = 0.33 at 30 days and P = 0.12 at 7 days).
Discussion
The current study demonstrates that LAAC can be successfully and relatively safely performed in an even wider variety of patients, both in terms of the greater variation of clinical settings than was seen in past clinical trials and in terms of a higher proportion of patients deemed unsuitable for OAT. The average CHADS 2 score of 2.8 and CHA 2 DS 2 -VASc score of 4.5 indicate a relatively higher risk of stroke than either the PROTECT AF (average CHADS 2 of 2.2 and CHA 2 DS 2 -VASc of 3.4) or PREVAIL (CHADS 2 score of 2.6 and CHA 2 DS 2 -VASc of 4.0) studies. In addition, 40% of EWOLUTION subjects had a HAS-BLED score of ≥3, compared with only 20% of PROTECT AF subjects and 30% of PREVAIL subjects. 12
The continuous improvements in implant techniques resulted in 98.5% successful WATCHMAN deployments, with 99.3% fulfilling the criteria for LAAC. The median number of devices used per procedure was only 1.07, while in 92.7% of patients only one device was needed, and 71.1% was deployed properly at the first attempt. These results compare favourably with all prior WATCHMAN LAAC trials.
The WATCMAN is not the only LAA closure device that has been studied in a real-world population. Tzikas et al . 13 recently published on a non-randomized registry with over 1000 patients treated with the AMPLATZER Cardiac Plug (ACP). The study methodology was different as it was retrospective observational, with different pre-specified procedural and major event endpoints, a difference in time windows of reporting, and without an event adjudication committee. The patient characteristics with regard to CHADS 2 , CHA 2 DS 2 -VASc, and HAS-BLED scores are very similar to those in EWOLUTION, which is reassuring in that these patients do represent the current target population in accordance with ESC guidelines. They found a 6.5% adverse event rate (68 major and other adverse events) during a procedure to 7-day follow-up of 1047 patients, compared with 2.8% in the present WATCHMAN study. Of the 63% of patients with TEE follow-up, the reported complete closure rate was 98.1% with some form of peri-device leak of 11.6%, which is fairly similar to the TEE data in our population.
The safety profile of EWOLUTION also compared favourably; pericardial effusion, procedural air embolism, acute stroke, and device embolism were all observed at or below levels reported in previous studies. In particular, the rate of procedural/device-related strokes was 0.9% in PROTECT AF 3 and 0.4% in PREVAIL, 14 compared with a rate of 0.1% through 30 days in this study. More generally, incidence of procedure- or device-related SAEs through 7 days occurred at a rate of 2.8%, compared with rates of 8.7% in PROTECT AF, 4.1% in the CAP registry, and 4.2% in PREVAIL. 12 The 30-day procedure- or device-related SAE rate was 3.6%. This demonstrates that such events can be effectively lowered with appropriate attention and training, but still highlights the inherent upfront risk of an invasive procedure. As these are acute and often manageable events for which patients are no longer practically at risk in longer-term follow-up, the risk/benefit consideration for the device compared with pharmacological therapy has a time-dependence. Appropriate patient selection remains mandatory, as patients who have many co-morbidities and limited expected life span may not expect benefit from LAAC given this time-dependence; subjects must have a sufficient life expectancy to profit from LAAC stroke prevention and avoidance of OAT.
Of great importance, EWOLUTION now provides a much larger body of data on the acute results of LAAC in over 600 patients deemed to be ineligible for OAT, with 738 patients not prescribed to OAT following the procedure. The much smaller prospective cohort study of 150 subjects, ASAP, cited an incidence of serious procedure- or device-related events of 8.7%. 3 The results of EWOLUTION, with a corresponding rate for all subjects of 2.8% at 7 days and 3.6% at 30 days ( Table 3 ), and among OAT ineligible subjects rates of 2.2% at 7 days and 2.9% at 30 days ( Figures 2 and 4 ), are encouraging in that it demonstrates that the maturation of the technology and implant technique has carried over into the OAT ineligible population. Furthermore, the nominal difference in event rates between OAT ineligible and eligible subjects was more pronounced for 30-day SAEs, independent of relatedness to the procedure or device. This finding must be taken with caution and may simply be due to less intense use of anticoagulation in VKA-ineligible patients. Though there are limitations of this post hoc analysis, this finding is consistent with the theory that some events such as groin bleeding or pericardial effusion that are ostensibly the result of the procedure may have increased likelihood because of OAT use, although this was not statistically different due to the low event rate. Supporting this is the finding of nominally lower rates of events for subjects with higher HAS-BLED scores. While many subjects are not eligible for OAT because of bleeding risk, this bleeding risk does not appear to confer LAAC procedural-related bleeding risk. All in all, 70% or more of this population did not use novel oral anticoagulants [(N)OACs] either pre- or post-procedure, as their physician deemed most of them ineligible. Therefore, even without LAA, this population with a mean CHADS-VASc score of 4.5 has a very high stroke risk (close to 7.5% per year 14 ) if left untreated. Whether the three strokes that occurred after implant were related to not using (N)OAC cannot be determined from the present data.
Figure 4.
Subgroup results—serious procedure-/device-related events through 7 days. The percentage (95% confidence interval) of the number of subjects experiencing events is displayed along the horizontal axis, separately for each subgroup defined by baseline characteristics. P -values are based on log-rank tests. OAT, oral anticoagulation therapy.
Limitations of this analysis on peri-procedural outcomes include the observational nature of the design and the relatively short follow-up of 30 days, while subgroup analyses were largely exploratory. The longer-term follow-up of stroke, embolism, and bleeding will provide further insights on the net clinical benefit of LAAC. While there is no control group included in the study, the past studies of the same device along with the ability to make risk adjustments via validated risk scores such as CHADS 2 , CHA 2 DS 2 -VASc, and HAS-BLED help provide comparative information on the therapy and quantify its use in a more general setting. Although EWOLUTION was set up as a prospective registry with clear pre-specified data collection, and MSG for event adjudication, and CRO monitoring, the study methods are not equal to those in prior RCTs, which may limit a direct comparison of outcome. Finally, an even larger study with more patients in particular subgroups of interest might have provided more power to detect even smaller differences in rates between groups.
Conclusion
The WATCHMAN device has a high success rate of LAA closure with low peri-procedural risk, even in patients with more co-morbidities and higher risk for stroke and bleeding. Improvement in implantation techniques has led to a consistent reduction of peri-procedural complications that were previously limiting the net clinical benefit of the procedure.
Authors’ contributions
K.S.: handled funding and supervision; L.V.A.B., B.S., T.B., H.S., C.T., E.T., T.S., S.K., Y.P., M.W.B.: acquired the data; L.V.A.B., B.S., T.B., H.S., C.T., E.T., K.S., M.W.B.: conceived and designed the research; L.V.A.B., K.S., M.B.: drafted the manuscript; L.V.A.B., B.S., T.B., H.S., C.T., E.T., T.S., S.K., Y.P., M.W.B: made critical revision of the manuscript for key intellectual content.
Funding
The study was funded by Boston Scientific Inc, Minneapolis, USA.
Conflict of interest: L.V.A.B. received consultancy fees BScI/Medtronic go to Cardiology Department. C.T. and T.R.B. received personal fees BScI. T.S. received personal fees BScI/SJM. M.W.B. received personal fees BScI/SJM/Biosense Webster/Johnson & Johnson. H.S. reports personal fees from Abbott, Aptus, Atrium, Biosense Webster, Boston Scientific, Carag, Cardiac Dimensions, CardioKinetix, CardioMEMS, Cardiox, Celonova, CGuard, Coherex, Comed B.V., Contego, Covidien, CSI, CVRx, ev3, FlowCardia, Gardia, Gore, GTIMD Medical, Guided Delivery Systems, Hemoteq, InSeal Medical, InspireMD, Kona Medical, Lumen Biomedical, Lifetech, Lutonix, Maya Medical, Medtronic, Occlutech, pfm Medical, Recor, Trireme, Trivascular, Valtech, Vascular Dynamics, Venus Medical, Veryan, Vessix, Cardiokinetix, Access Closure, Coherex, SMT.
Acknowledgements
The following investigators and institutions participated in the EWOLUTION registry. Investigators are listed after centres in alphabetical order: Al Qasimi Hospital: Arif Al Nooryani, Asklepios Klinik Saint Georg: Felix Meincke, Asklepios Klinik Weissenfels: Sven Mobius-Winkler, ASL TO 4 Ospedale di Cirie: Gaetano Senatore, Beaumont Hospital: David Foley, Cardioangiologisches Centrum Bethanien: Boris Schmidt, CHRU de Lille: François Brigadeau, CHU Grenoble Hopital Michallon: Pascal Defaye, CHU Henri Mondor: Emmanuel Teiger, CHU La Timone Hospital: Jean-Louis Bonnet, Dominikus-Krankenhaus: Christof Wald, Elisabeth Krankenhaus Essen: Thomas Schmitz, Erasmus MC – University Medical Center Rotterdam: Tamas Szili-Torok, Evangelisches Krankenhaus Bielefeld: Wladimir Tschishow, Fondazione Centro San Raffaele: Patrizio Mazzone, Freeman Hospital: David Crossland, Herzkatheter Asklepios Wandsbek: Martin Bergmann, Hôpital Bichat: Alec Vahanian, Hospital Clinico Salamanca: Ignacio Cruz-Gonzalez, Hospitaux du Haut Leveque: Jean-Benoit Thambo, Johannes Gutenberg Universitaet Mainz: Tommaso Gori, John Radcliffe Infirmary Oxford II: Timothy Betts, King Fahed Medical City Prince Salman Cardiac Center: Faisal Al Smadi, Klinikum Neuperlach: Harald Mudra, Krankenhaus Barmherzige Bruder: Robin Molitoris, Medisch Centrum Leeuwarden: Richard Folkeringa, Medisch Spectrum Twente: Yorick Stevenhagen, NCN Nouvelles Cliniques Nantaises: Daniel Gras, Onze Lieve Vrouw Ziekenhuis: Tom De Potter, Ospedale Ferrarotto: Corrado Tamburino, Ospedale Sacro Cuore Don Calabria: Giulio Molon, Regional Vascular Center: Vladimir Protopopov, Royal Victoria Hospital: Mark Spence, Samodzielny Publiczny Szpital: Marek Grygier, Santa Maria: Eduardo Infante Oliveira, St Antonius Ziekenhuis: Lucas Boersma, St. Katharinen Krankenhaus: Horst Sievert, State Cardiology Research Center: Evgeny Merkulov, State Research Institute of Circulation Pathology: Evgeny Pokushalov, Szpital Uniwersytecki: Adam Sukiennik, The Brompton Hospital: Tom Wong, Universitatsmedizin Greifswald: Mathias Busch, University Berlin, Charite Virchow Standort: Leif-Hendrik Boldt, University KH Bonn: Georg Nickenig, University Leipzig: Norbert Klein, Vivantes Klinikum Am Urban: Iskandar Atmowihardjo, Vivantes Klinikum im Friedrichshain: Stephan Kische.
References
- 1. Blackshear J , Odell J . Appendage obliteration to reduce stroke in cardiac surgical patients with AF . Ann Thorac Surg 1996. ; 61 : 755 – 759 . [DOI] [PubMed] [Google Scholar]
- 2. Holmes DR , Reddy VY , Doshi SK , Sievert H , Buchbinder M , Neuzil P , Huber K , Halperin J , Holmes D , on behalf of the PROTECT AF Investigators . Percutaneous closure of the left atrial appendage versus warfarin therapy for prevention of stroke in patients with atrial fibrillation: a randomised non-inferiority trial . Lancet 2009. ; 374 : 534 – 542 . [DOI] [PubMed] [Google Scholar]
- 3. Holmes DR Jr , Kar S , Price MJ , Whisenant B , Sievert H , Doshi SK , Huber K , Reddy VY . Prospective randomized evaluation of the WATCHMAN left atrial appendage closure device in patients with atrial fibrillation versus long-term warfarin therapy: the PREVAIL trial . J Am Coll Cardiol 2014. ; 64 : 1 – 12 . [DOI] [PubMed] [Google Scholar]
- 4. Reddy VY , Sievert H , Halperin J , Doshi SK , Buchbinder M , Neuzil P , Huber K , Whisenant B , Kar S , Swarup V , Gordon N , Holmes D , for the PROTECT AF Steering Committee and Investigators . Percutaneous left atrial appendage closure vs warfarin for atrial fibrillation: a randomized clinical trial . JAMA 2014. ; 312 : 1988 – 1998 . [DOI] [PubMed] [Google Scholar]
- 5. Camm AJ , Lip GY , De Caterina S , Savelieva I , Atar D , Hohnloser SH , Hindricks G , Kirchhof P , ESC Committee for Practice Guidelines (CPG) . 2012 Focused update of the ESC guidelines for the management of atrial fibrillation: an update of the 2010 ESC guidelines for the management of atrial fibrillation: developed with the special contribution of the European Heart Rhythm Association . Eur Heart J 2012. ; 33 : 2719 – 2747 . [DOI] [PubMed] [Google Scholar]
- 6. Meier B , Blaauw Y , Khattab AA , Lewalter T , Sievert H , Tondo C , Glikson M . EHRA/EAPCI expert consensus statement on catheter-based left atrial appendage occlusion . EuroIntervention 2014. ; 10 : 1109 – 1125 . [DOI] [PubMed] [Google Scholar]
- 7. Windecker S , Kolh P , Alfonso F , Collet JP , Cremer J , Falk V , Filippatos G , Hamm C , Head S , Juni P , Kappetein P , Kastrati A , Knuuti J , Landmesser U , Laufer G , Neumann FJ , Richter D , Schauerte P , Sousa Uva M , Stefanini G , Taggart DP , Torracca L , Valgimigli M , Wijns W , Witkowski A . 2014 ESC/EACTS Guidelines on myocardial revascularization . Eur Heart J 2014. ; 35 : 2541 – 2619 . [DOI] [PubMed] [Google Scholar]
- 8. Meschia JF , Bushnell C , Boden-Albala B , Braun LT , Greenberg S , Horvath SE , Iadecola C , Jauch E , Moore W , Wilson JA , on behalf of the American Heart Association Stroke Council , Council on Cardiovascular and Stroke Nursing , Council on Clinical Cardiology , Council on Functional Genomics and Translational Biology , and Council on Hypertension . Guidelines for the primary prevention of stroke: a statement for healthcare 21 professionals from the American Heart Association/American Stroke Association . Stroke 2014. ; 45 : 3754 – 3832 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Boersma L , Schmidt B , Betts T , Sievert H , Tamburino C , Teiger E , Stein K , Bergmann M . EWOLUTION: design of a registry to evaluate real-world clinical outcomes in patients with AF and high stroke risk-treated with the WATCHMAN left atrial appendage closure technology . Catheter Cardiovasc Interv 2016. ; in press . [DOI] [PubMed] [Google Scholar]
- 10. Mehran R , Rao SV , Bhatt DL , Gibson CM , Caixeta A , Eikelboom J , Kaul S , Wiviott SD , Menon V , Nikolsky E , Serebruany V , Valgimigli M , Vranckx P , Taggart D , Sabik JF , Cutlip DE , Krucoff MW , Ohman EM , Steg PG , White H . Standardized bleeding definitions for cardiovascular clinical trials: a consensus report from the Bleeding Academic Research Consortium . Circulation 2011. ; 123 : 2736 – 2747 . [DOI] [PubMed] [Google Scholar]
- 11. Leon MB , Piazza N , Nikolsky E , Blackstone EH , Cutlip DE , Kappetein AP , Krucoff MW , Mack M , Mehran R , Miller C , Morel MA , Petersen J , Popma JJ , Takkenberg JJ , Vahanian A , van Es GA , Vranckx P , Webb JG , Windecker S , Serruys PW . Standardized endpoint definitions for transcatheter aortic valve implantation clinical trials: a consensus report from the Valve Academic Research Consortium . Eur Heart J 2011. ; 32 : 205 – 217 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Reddy VY , Holmes D , Doshi SK , Neuzil P , Kar S . Safety of percutaneous left atrial appendage closure: results from the WATCHMAN left atrial appendage system for embolic protection in patients with AF (PROTECT AF) clinical trial and the continued access registry . Circulation 2011. ; 123 : 417 – 424 . [DOI] [PubMed] [Google Scholar]
- 13. Tzikas A , Shakir S , Gafoor S , Omran H , Berti S , Santoro G , Kefer J , Landmesser U , Nielsen-Kudsk JE , Cruz-Gonzalez I , Sievert H , Tichelbäcker T , Kanagaratnam P , Nietlispach F , Aminian A , Kasch F , Freixa X , Danna P , Rezzagh M , Vermeersch P , Stock F , Stolcova M , Costa M , Ibrahim R , Schillinger W , Meier B , Park JW . Left atrial appendage occlusion for stroke prevention in atrial fibrillation: multicentre experience with the AMPLATZER Cardiac Plug . EuroIntervention 2015. ; 10 . 10.4244/EIJY15M01_06 . [DOI] [PubMed] [Google Scholar]
- 14. Reddy VY , Möbius-Winkler S , Miller MA , Neuzil P , Schuler G , Wiebe J , Sick P , Sievert H . Left atrial appendage closure with the WATCHMAN device in patients with a contraindication for oral anticoagulation. The ASAP study (ASA Plavix Feasibility Study with WATCHMAN Left Atrial Appendage Closure Technology) . J Am Coll Cardiol 2013. ; 61 : 2551 – 2556 . [DOI] [PubMed] [Google Scholar]