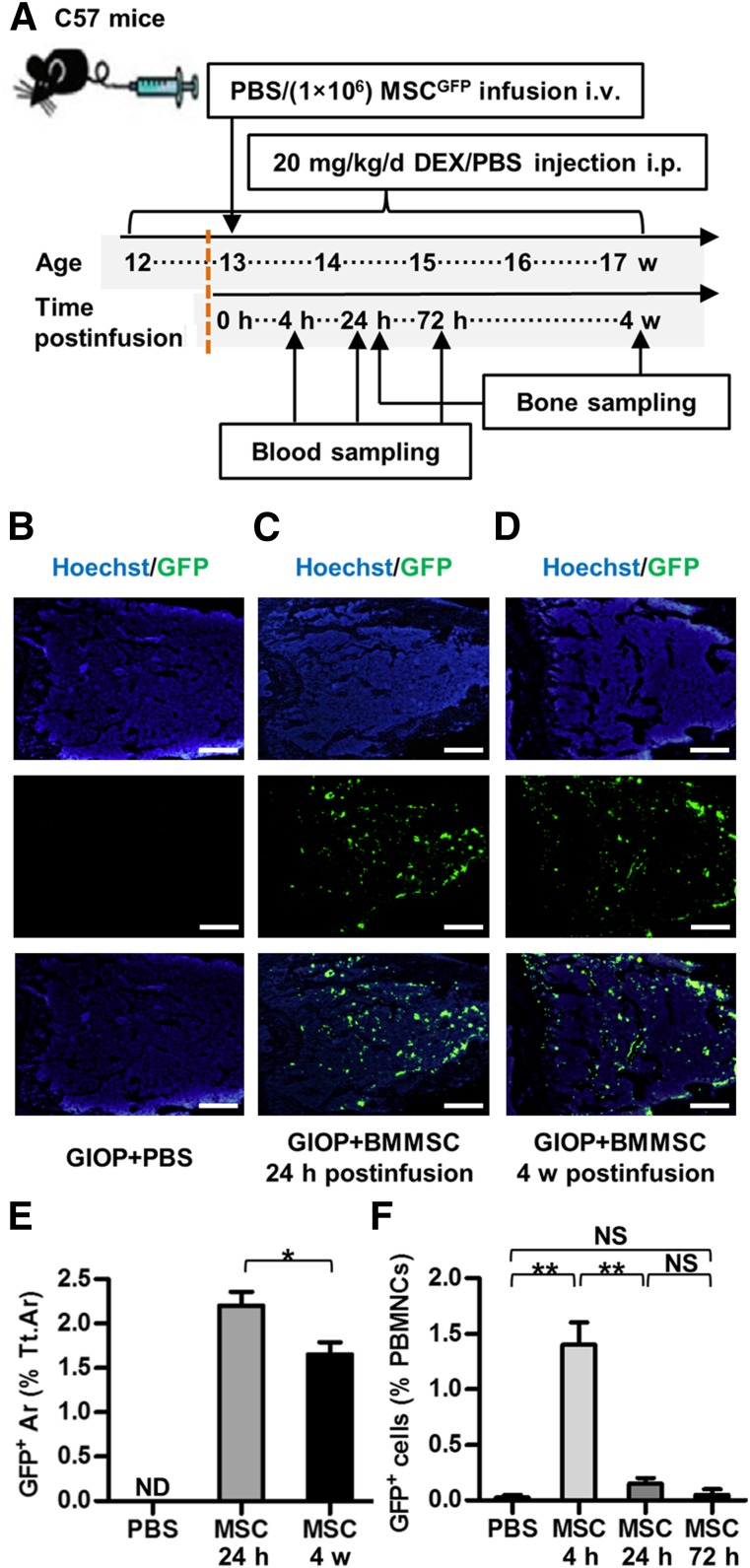
Figure 4.
Study design of Experiment 3 and inhabitation of donor BMMSCsGFP in recipient bone marrow. (A): Study design of Experiment 3 for cell fate investigation. Blood and bone were sampled at indicated time points. (B–D): Representative images demonstrating Hoechst staining for total cells (blue), GFP immunofluorescence for donor BMMSCsGFP (green), and merged labeling. Scale bars: 500 μm. (E): Corresponding parameter showing inhabitation of donor BMMSCsGFP in recipient bone marrow for at least 4 weeks. (F): Flow cytometric analysis of PBMNCs showing clearance of donor BMMSCsGFP after 24 hours. Data represent mean ± SEM; n = 4 per group. ∗, p < .05; ∗∗, p < .01. Abbreviations: Ar, area; BMMSC, bone marrow-derived mesenchymal stem cell; Cont, control; d, day; DEX, dexamethasone; GIOP, glucocorticoid-induced osteoporosis; GFP, green fluorescent protein; h, hours; i.p., intraperitoneally; i.v., intravenously; MSC, mesenchymal stem cell; ND, not detected; NS, not significant; PBMNCs, peripheral blood mononuclear cells; PBS, phosphate-buffered saline; Tt.Ar, total area; w, weeks.