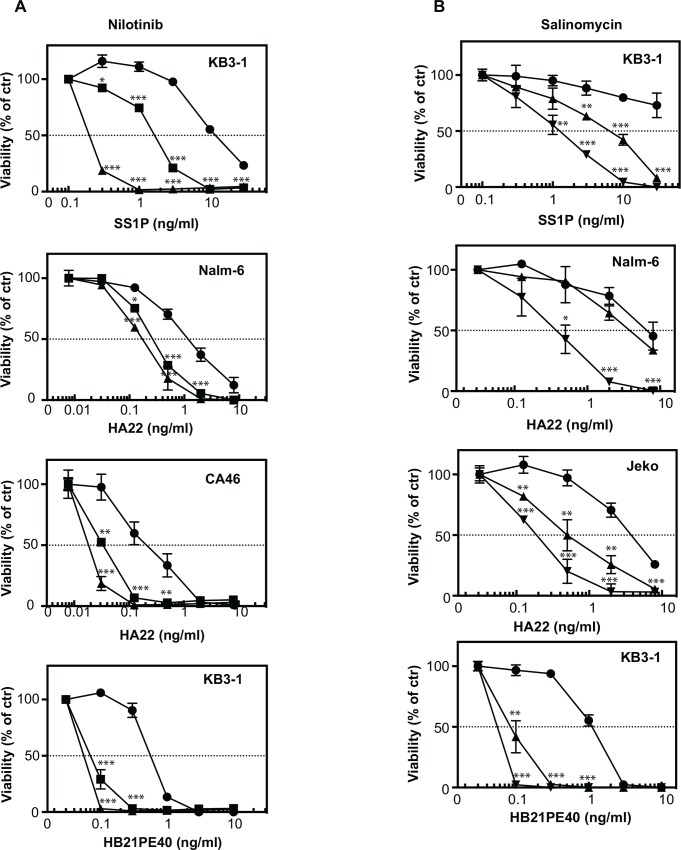
Fig 2. Confirmation of enhancer activity for nilotinib and salinomycin combinations.
Cells were treated for 72 hours in a dose dependent manner with immunotoxins in the absence (closed circle) or in the presence of different concentrations of nilotinib (A) or they were treated for 48 hours in a dose dependent manner with immunotoxins in the absence (closed circle) or in the presence of different concentrations of salinomycin (B). Nilotinib was tested at 1 μM (closed square) and 2 μM (closed triangle) Salinomycin was tested at 0.05 μM (closed tringle) and 0.1 μM (inverted closed triangle) in Nalm-6 and Jeko cells with HA22 and at 0.025 μM (closed triangle) and 0.05 μM (inverted closed triangle) in KB3-1 cells with SS1P and HB21PE40. Data are from at least two independent experiments in triplicate. Error bars display SD value. Two tailed unpaired t-tests were performed at each concentration point. Two tailed unpaired t-tests were performed comparing the viability at each concentration of immunotoxin alone versus the same concentrations plus the drug with p<0.05 significant. *p<0.05, **p<0.01, ***p<0.001.