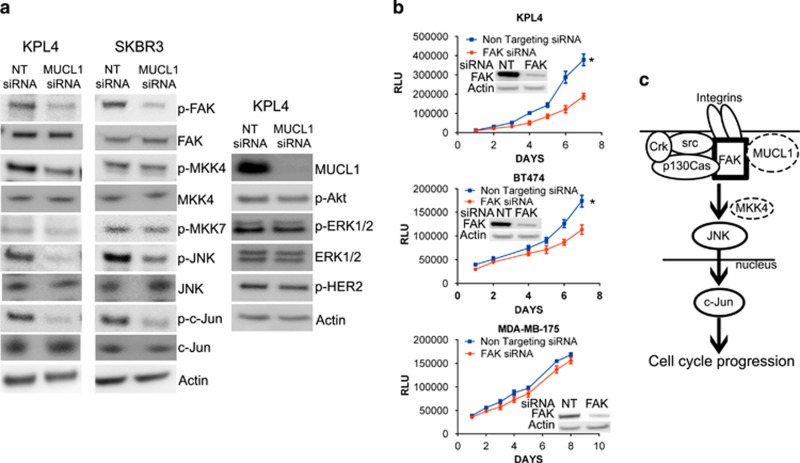
Figure 7.
(a) KPL4 and SKBR3 cells were transiently transfected with MUCL1 siRNA or NT control siRNA and lysed for western blotting 48 h later. Immunoblot analysis showed a decrease in the phosphorylation of FAK, JNK and c-Jun in MUCL1 cells as compared with control cells, and phosphorylated MKK4 was decreased in KPL4 cells only. No changes were detected in activation of MKK7, Akt, ERK1/2 or HER2. β-Actin was used as a loading control. (b) Breast cancer cells were transfected with FAK siRNA or NT control siRNA. Cell proliferation was assessed by Cell Titer Glo Assay each day for 1 week. Mean ±s.d. is shown (n=10). *P<0.001 for difference of growth rate. FAK knockdown was confirmed by western blotting. (c) Proposed model depicting MUCL1-mediated FAK activation and signaling to downstream JNK. A potential interaction between MUCL1 with FAK is likely to be intracellular. Following integrin engagement, JNK activation requires association of FAK with a Src kinase and p130Cas, and the recruitment of Crk. The activation of JNK may be through MKK4 or other mediators depending on the cell type. On stimulation by JNK, c-Jun translocates to the nucleus and mediates G1/S phase transition leading to cell proliferation. The experiments were repeated twice with similar results.