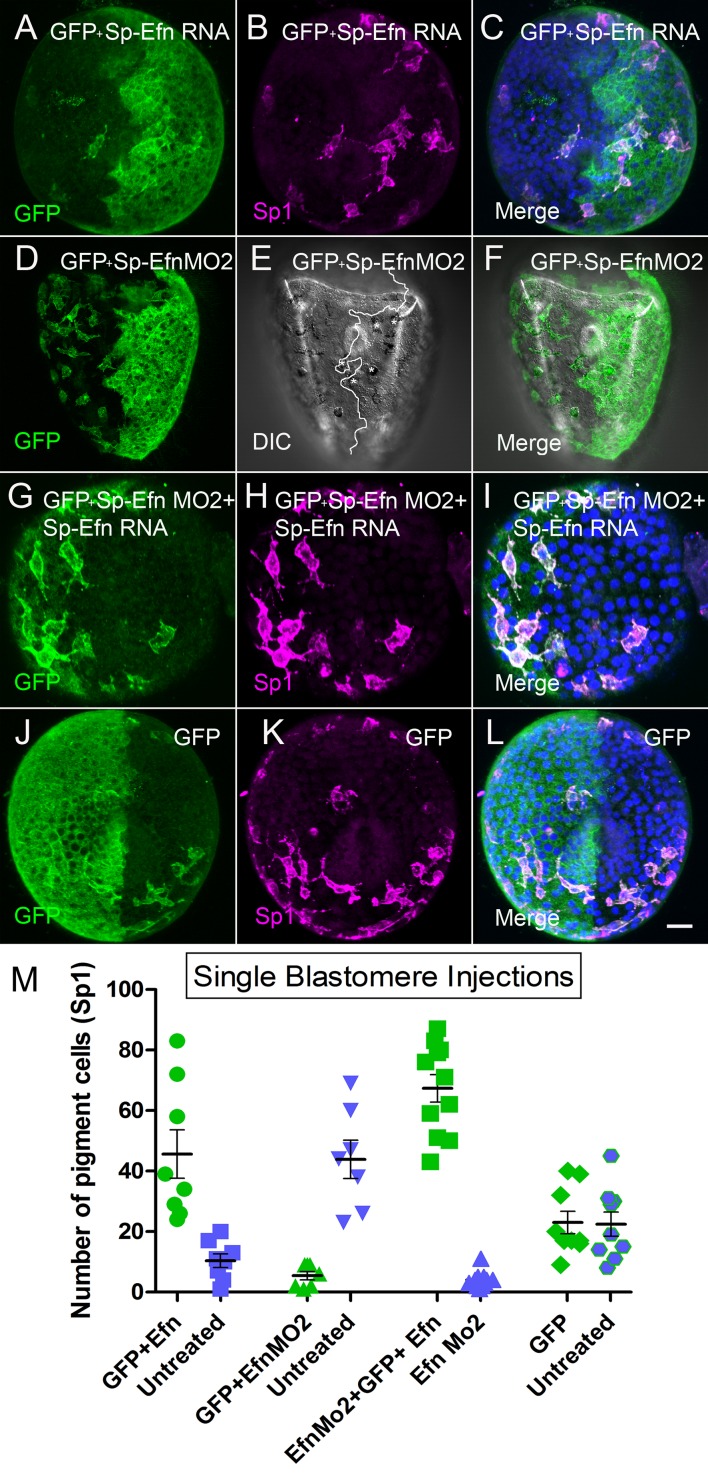
Figure 5. Altering the abundance of Sp-Efn in half embryos by injecting a single blastomere of 2-cell embryos indicates pigmented immunocytes insert preferentially in ectoderm expressing Sp-Efn and high levels of expression of Sp-Efn enhances pigment cell insertion.
(A–C) Maximum intensity projection of an embryo in which half of the specimen co-expresses membrane GFP and Sp-Efn (MeOH fixation). Sp1 reveals the distribution of immunocytes. Note that a subset of the immunocytes expresses GFP. Most of the immunocytes are either inserted in the half expressing Sp-Efn or extend contacts to that half of the embryo. (D–F) Through focus projection of a living embryo that was co-injected in one blastomere with GFP and SpEfn MO2. In the DIC image immunocytes can be identified by their pigment and the white line demarks the interface between ectoderm containing morpholino (anatomical left) and untreated ectoderm (anatomical right). A subset of the immunocytes express GFP. Several pigmented immunocytes are associated with the interface between the to domains of ectoderm, those marked with * project processes to the untreated, Sp-Efn expressing, ectoderm. (G–I) Embryo from an egg that was injected with Sp-EfnMO2 to suppress Sp-Efn expression throughout the embryo (MeOH fixation). Once the egg had cleaved, one blastomere was injected with Sp-Efn RNA. The pigmented immunocytes are almost exclusively inserted in the half of the embryo expressing Sp-Efn. (J–L) Control embryos were injected with mGFP RNA only and the distribution of pigmented immunocytes can be seen to be unaffected (MeOH fixation). (M) Quantification of the distribution of pigmented immunocytes from the experiments depicted above (A–L). For each treatment (X axis) there is an injected half (green) and an uninjected half (blue). The number of pigmented immunocytes inserted in ectoderm of the injected half, or the uninjected half was determined for each embryo (72 hr). Bar = 10 µm
DOI: http://dx.doi.org/10.7554/eLife.16000.018