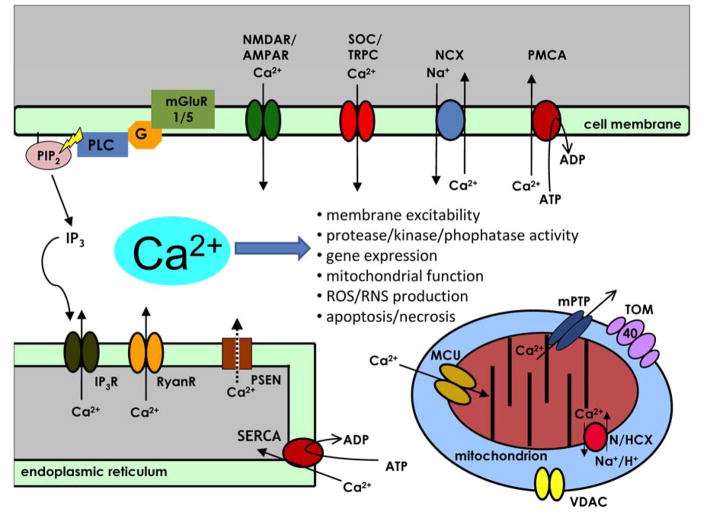
Figure 1. The compartmentalization of intracellular Ca2+ signalling in neurons and AD pathogenesis.
Calcium (Ca2+) is a key regulator of many neuronal processes and serves as the critical link between environmental stimuli and the intracellular effectors that result in a physiological response (Berridge, 1998). Gene expression, protein processing, ATP production, neurotransmitter release, action potential generation, modulation of membrane excitability, short-term and long-term synaptic plasticity, neurite outgrowth and control of cell death mechanisms are Ca2+-regulated processes that are imperative for neuronal function. The proteins that bind free Ca2+, such as calmodulin (CaM), and activate Ca2+-dependent cellular processes are expressed in membrane enclosed compartments such as the cytoplasm, the endoplasmic reticulum (ER) or the mitochondria (mt). The concentration of free Ca2+ ([Ca2+]) and the spatio-temporal pattern of Ca2+ microdomains determines the activation of particular cellular processes (Berridge, 2006). Thus, the [Ca2+] in each compartment is tightly regulated. Plasma membrane Ca2+ ATPases (PMCA), sodium/calcium exchangers (NCX) and sarco-/endoplasmic reticulum Ca2+ ATPases (SERCA) set up an electrochemical gradient which, upon neuronal activation, Ca2+ ions can passively move between cellular compartments through voltage- and/or ligand-gated channels. Calcium influx from the extracellular matrix can happen through voltage-gated Ca2+ channels (VGCC), N-methyl-D-aspartate receptors (NMDAR), α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors (AMPAR), store-operated channels (SOC, eg. transent receptor potential channels (TRPC)). Calcium efflux from intracellular ER stores is mediated by inositol 1,4,5-trisphosphate receptors (IP3R), ryanodine receptors (RyanR) and presenilins (PSEN) which facilitate “ER Ca2+ leak” (Tu et al., 2006). Mitochondria participate in Ca2+ signaling by taking up Ca2+ from cytosolic or ER microdomains across the outer mitochondrial membrane (OMM) through unknown mechanisms, likely through the voltage-dependent anion channel (VDAC) into the inner mitochondrial membrane (IMM) lumen through the mitochondrial Ca2+ uniporter (MCU). Recently, a Ca2+/H+ anti-porter (leucine zipper EF-hand–containing transmembrane protein 1, Letm1) that transports Ca2+ from the cytosol into the IMM lumen in was identified in HeLa cells (Jiang et al., 2009) but its function in neurons is unknown. Polymorphisms in TOMM40 gene encoding outer mitochondrial membrane component of the TOM complex have been linked with the probability of developing late-onset AD (Potkin et al., 2009; Roses et al., 2009; Shen et al., 2010; Takei et al., 2009). Calcium equilibrium is maintained along the IMM by NCX or hydrogen/calcium exchangers (HCX). Opening of the mitochondrial permeability transition pore (mtPTP) allows large efflux of Ca2+ from the IMM lumen and is often a trigger for the cell death signalling cascade (Giacomello et al., 2007). Under normal circumstances following neuronal stimulation, active Ca2+ transport returns [Ca2+] in each compartment to homeostatic levels. Both active and passive Ca2+ handling mechanisms are subject to regulation, in fact, Ca2+ itself is an important regulator of Ca2+ channel activity.