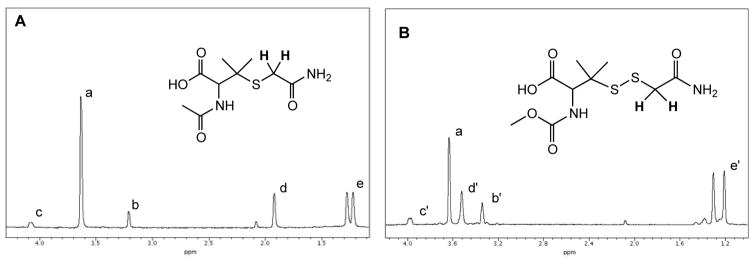
Figure 5.
1H-NMR Spectra of the reaction of IAA with (A) NAP and (B) MCPP. IAA was present at 10-fold excess. Methylene protons on IAA resonate at approx. 3.62 ppm (a). Methylene protons of the IAA adducts (shown in bold) resonate upfield at approx. 3.2 ppm for the NAP adduct (b) and 3.2 ppm for the MCPP (b’) adduct. Note: for the NAP adduct (A) the methine (CH) proton resonates at 4.1 ppm (c), the acetyl methyl protons (CH3) resonates at 1.9 ppm (d) and the diastereotopic gem-dimethyl protons (CH3,CH3) resonate at 1.2 – 1.3 ppm (e); for the MCPP adduct (B) the methine proton (CH) resonates at 4 ppm (c’), the carbonyl O-methyl protons (OCH3) resonate at approx. 3.5 ppm (d’) and the gem-dimethyl protons (CH3,CH3) resonate at 1.2–1.3 ppm (e’).