Figure 1.
Stepping patterns of MyoVc, MyoVa, and a chimeric motor on single actin filaments.
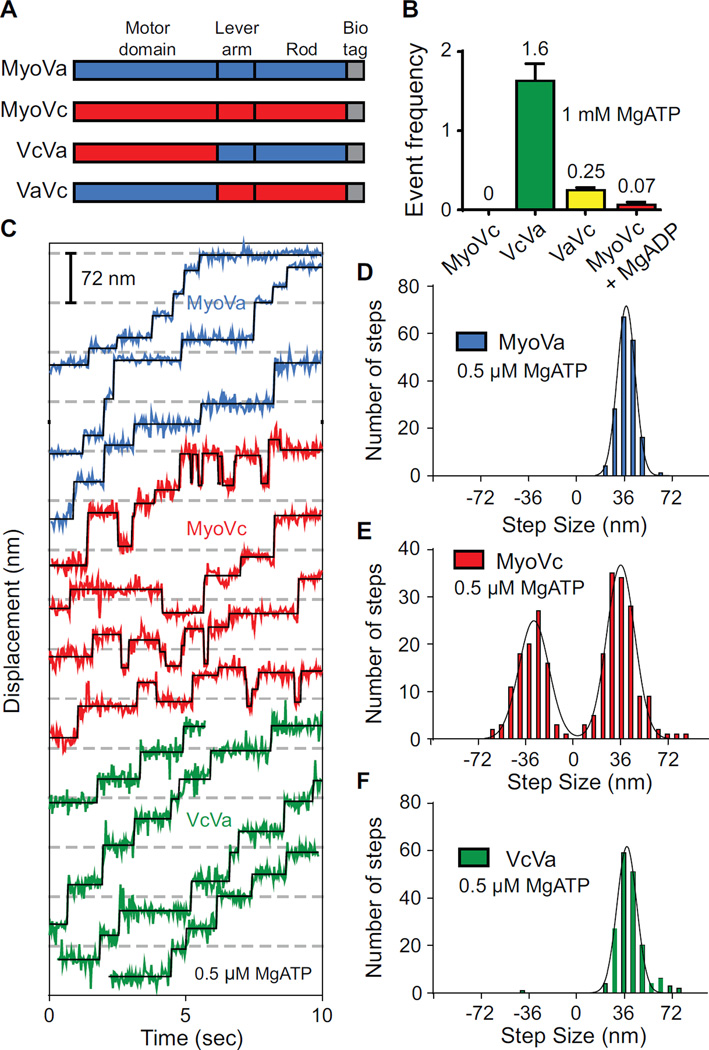
(A) Schematic showing truncated MyoVa-HMM and MyoVc-HMM constructs used here. MyoVa-HMM (MyoVa, blue) and MyoVc-HMM (MyoVc, red) contain three domains: motor domain, lever arm and rod. The chimeric construct, VcVa contains the motor domain of MyoVc and the lever arm and rod of MyoVa. VaVc contains the motor domain of MyoVa and the lever arm and rod of MyoVc. Constructs contain a C-terminal biotin tag (gray) for attachment to streptavidin-Qdots for visualizing movement on different actin tracks.
(B) Event frequencies (µM myosin*µm actin*sec)−1 of MyoVc (0), VcVa (green, 1.6 ± 0.22), VaVc (yellow, 0.25 ± 0.033) and MyoVc + 200 µM MgADP (red, 0.067 ± 0.032) on single actin filaments. Single motor motility was done at 1 mM MgATP and 25 mM KCl.
(C) Representative displacement versus time data for MyoVa (blue), MyoVc (red) and the VcVa chimera (green) at 0.5 µM MgATP. Steps were fitted to the raw data using a step-finding algorithm (black lines). Displacements were determined to be steps only if they persisted for at least two frames (67 ms).
(D–F) Step size histograms of (D) MyoVa, (E) MyoVc and (F) a VcVa chimeric construct. Histograms were fit to single or multiple Gaussian distributions to determine the average step sizes ± SD for each construct; MyoVa, 37nm ± 6.8 (N = 173); MyoVc, 33nm ± 10.6 and -33nm ± 11.4 (N = 247); VcVa, 38nm ± 7.5 (N = 177).
See also Figures S1–S4 and Movie S1.