Abstract
Intracellular calcium (Ca2+) levels play a vital role in regulating cellular fate. The coordination and interrelation among the cellular organelles, mainly the intracellular Ca2+ stores in endoplasmic reticulum (ER), are crucial in maintaining cytosolic Ca2+ levels and in general cellular homeostasis. Moreover, maintaining Ca2+ homeostasis is essential for regulating diverse and sometimes opposing processes such as cell survival and cell death in disease conditions such as, neurodegeneration, cancer and aging. Ca2+ is able to regulate opposing functions by either regulating the cellular “self-eating” phenomenon of autophagy to promote cell survival or by regulating the programmed cell death process of apoptosis. Autophagy is also important for cell survival especially after induction of ER stress and association between ER stress and autophagy may have relevance to numerous diseases. Moreover, a multitude of evidence is emerging that the functional regulation of TRP channels, their unique localization, and their interaction with other Ca2+-sensing elements define these diverse regulatory pathways. It is this unique function which allows individual TRP channels to contribute differently in the regulation of cell fate and, in turn, determines the precise effect of modulating Ca2+ signaling via the particular channel. Thus, in this review we have focused on the aspects of TRP channel localization and function (Ca2+ signaling) that affects the ER stress and autophagic process.
Keywords: Ca2+, TRPC1 TRPML1, TRPML3, TRPV, autophagy, ER stress
Introduction
Disease conditions, such as cancer progression, are closely related to dysregulations of the cell cycle and are accompanied by enhanced cell proliferation and/or suppression of apoptosis leading to cell death [1-3]. In contrast, degenerative processes are initiated by either enhanced cell death and/or suppression of cell proliferation. The key factors between these opposing biological processes often lead to a perturbed balance between the processes of proliferation, autophagy, and apoptosis [1, 4]. Ca2+ is one of the most important regulators of cell survival/death processes. As a second messenger, Ca2+ is able to activate or inactivate various regulatory proteins such as enzymes, transcriptional factors, or molecular chaperones. Importantly, Ca2+ signaling has been shown to regulate cellular processes such as cell proliferation, survival, migration, invasion, motility, autophagy and apoptosis [5-7]. The adaptability of Ca2+ signaling in regulating these diverse functions derives from the multitude of components that can be divided into several distinct processes. First, the response to a stimulus creates Ca2+-mobilizing signals that in turn releases Ca2+ from internal stores, such as the endoplasmic reticulum (ER). Second, ER Ca2+ release initiates Ca2+ entry, via numerous membrane channels like the transient receptor potential (TRP) channels [8, 9]. Third, Ca2+ subsequently functions as a second messenger to activate a cascade of Ca2+-sensitive processes, before a series of mechanisms relying on Ca2+ pumps and ion exchangers that removes Ca2+ from the cytoplasm thereby restoring the resting state (Figure 1A). Each of the above described stages can be accurately controlled to create Ca2+ gradients which vary considerably in their spatial and temporal patterns.
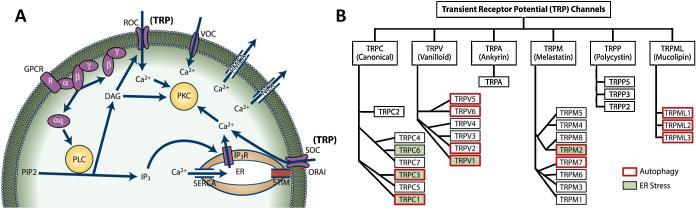
Figure 1. Ca2+ signaling and TRP channel classification.
Schematic diagram for the common Ca2+ signaling pathways are shown in (A). Cells have several mechanisms for regulating cytosolic Ca2+ concentration, but not necessarily all of the mechanism shown here are present in one single cell type. The activation of G coupled protein receptor complex dissociates and activates the enzyme PLC which catalyzes the dissociation of PIP2 to form DAG and IP3. DAG activates receptor-operated channels (ROC). IP3 binds to its receptors in the ER (IP3R) resulting in the release of the stored Ca2+ from the ER. Emptying of the ER Ca2+ activates the STIM protein to translocate to the PM and binds to Ca2+ release-activated Ca2+ channel protein (ORAI), thereby activating store-operated channels (SOC). TRP channels can act as a ROC and SOC. Increased Ca2+ in the cytosol is pumped out by PMCA and NCX. SERCA refills the stores. DAG and Ca2+ activate PKC which results in various downstream effects. Summary of the mammalian (human) TRP channel superfamily. TRPML (mucolipin), TRPP (polycystin), TRPM (melastatin), TRPA (ankyrin), TRPV (vanilloid) and TRPC (canonical) are shown in (B)
Modulation of Ca2+ permeable channels expression/function also affects intracellular Ca2+ concentrations and consequently Ca2+ dependent processes, such as cell proliferation, ER stress, apoptosis, and autophagy [1, 10, 11]. Ca2+ permeable channels, including families of transient receptor potential (TRP) channels, Orai's, voltage-gated Ca2+ channels, two-pore Ca2+ channels, mitochondrial Ca2+ uniporter, IP3 and ryanodine receptors have all been identified to contribute towards changes in intracellular Ca2+ ([Ca2+]i) [7, 8, 12, 13]. Among all the proteins that modulate Ca2+ signaling, the TRP family are the most diverse and have been shown to regulate various Ca2+-dependent physiological processes in different cell types [8] (Figure 1B).
The ER is the main source of intracellular Ca2+ and is involved in the synthesis of many macromolecules such as proteins, unsaturated fatty acids, and sterols. The most important function for ER is to regulate protein synthesis, their translocation, proper folding and modulating post-translational modifications, which are all dependent on intracellular Ca2+ concentrations. The ER then transports newly synthesized proteins to the Golgi apparatus and ultimately to the vesicles for secretion or display on the plasma membrane surface, a process that is also dependent on intracellular Ca2+ concentrations. Stress to the ER triggered by the disruption of Ca2+ homeostasis, disturbs folding of proteins and causes a buildup of unfolded/misfolded protein in the ER lumen, thus activating the unfolded protein response (UPR) pathway [14]. The purpose of the UPR pathway is to facilitate protein folding and return the ER to homeostasis. However, if homeostasis is not achieved the UPR pathway may eliminate the cell through apoptosis [15], but recent research suggest that this is not the only known cell fate.
UPR is not the only triggered action by ER stress, induction of autophagy can work in conjunction or independently of UPR to help the cell cope with ER stress by removal of misfolded proteins; however if UPR persists it can also assist in cellular death [16]. Studies have also shown that autophagy is necessary for cell survival especially after ER stress [17]. Ca2+ plays a vital role in regulating ER stress and autophagy, and also within the cross-talk between these two processes. Members of the Ca2+-permeable non-selective cation TRP family have been shown to mediate cellular Ca2+ homeostasis and inhibit ER stress through mechanisms which may also lead to autophagy. Importantly, Ca2+ is likely to have different regulatory effects on autophagy, depending on the spatial and sequential parameters of Ca2+ signaling proteins, nutrient and growth factor availability, as well as in various pathological conditions like cancer, inflammation, neurodegenerative disorders etc. [18, 19]. The role of transient potential (TRP) channel for cell survival and apoptosis is widely recognized, whereas the information about the significance of these channels in regulating autophagy and ER stress is still limited. In the present review we will provide an overview of the literature on the role of the Ca2+ permeable TRP channels in the regulation of ER stress and autophagy.
1. Autophagy
Autophagy is a cellular process responsible for the delivery of proteins or organelles to lysosomes for its degradation. When cells encounter stressful situations, they can either try to survive under these conditions via a very beneficial process called autophagy, or by activating a programmed cell death program through the process of apoptosis [11, 18, 20]. The word autophagy is derived from the Greek roots “auto” (self) and “phagy” (eating) and broadly refers to the cellular catabolic processes in which target material is transported to lysosomes for degradation. Autophagy is also an important pathway for the clearance of pathogens thereby also indirectly regulating cell survival [21]. Till date, three types of autophagy exist; macroautophagy, microautophagy and chaperone-mediated autophagy (CMA) (Figure 2) [18, 22]. Cellular stress conditions including nutrient starvation, hypoxia conditions, invading microbes, and tumor formation, have been shown to induce autophagy and allows cell survival in these stressful or pathological situations [23]. In addition, autophagy also recycles existing cytoplasmic components that are required to sustain vital cellular functions [24]. Although the precise mechanism as to how autophagy is initiated is not well understood, many of the genes first identified in yeast that are involved in autophagy have orthologues in other eukaryotes including human homologues [22, 25]. Presence of similar genes in all organisms suggests that autophagy might be a phenomenon that is evolutionally conserved and is essential for cell survival. Additionally, since autophagy delivers a fresh pool of amino acids and other essential molecules to the cell, initiation of autophagy is highly beneficial. In particular, autophagy is advantageous during nutritional stress situations or tissue remodeling during development and embryogenesis [22]. Although autophagy and apoptosis are mechanistically different cellular processes, there are some common regulatory proteins that intervene in both of them, such as the anti-apoptotic/anti-autophagy regulators Bcl-2 and Bcl-XL and Ca2+ signaling.
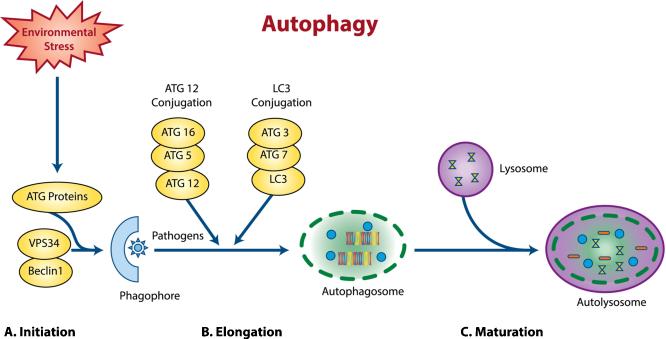
Figure 2. Basic macroautophagy process.
Shows a schematic diagram of the basic autophagy process. This process is divided into three processes of a) Initiation b) Elongation and c) Maturation. On stimulation with external environmental stimulus like cells stress, nutrient depletion, hypoxia, etc. the initiation process of autophagy starts with the activation of autophagy-related proteins (ATG) and activation of a complex of class III phoshoinositide 3-kinase VPS34 and beclin 1. The elongation and shape of the autophagosome are controlled by two protein conjugation system, the ATG12 conjugation and LC3 conjugation. During maturation the lysosomes associated autophagosomes are degraded into autolysosomes.
1.1 Molecular types of autophagy
Macroautophagy is the best studied form of autophagy and is characterized by the formation of double membrane vesicles called autophagosomes. The process of autophagosomes formation consists of several stages, namely initiation, elongation and maturation, and fusion [22] (Figure 2). Microautophagy implies direct delivery through the invagination and fission of the lysosomal membrane while CMA delivers material to lysosomes with help of chaperones. However, unlike macroautophagy, microautophagy does not appear to be related to the cellular adaptation to nutrient deprivation. [18, 24, 26, 27]. In chaperone-mediated autophagy the substrates have a pentapeptide lysosome-targeting motif (KFERQ) that is recognized by a complex of chaperone proteins and target the complex to lysosomal membrane. Given that autophagy has been linked to inhibit several diseases, like neurodegeneration, diabetes and infectious diseases as well as promotion of some cancer especially chemoresistance (as reviewed in [28], [29] and [30]); the linkages between ER stress and autophagy may also have relevance to several of these diseases.
1.2 Calcium and its role in autophagy
Intracellular Ca2+ plays an important role of both basal [31] and induced [32] autophagy. The first evidence on Ca2+ dependent regulation of autophagy was shown by Gordon et al.,1993 in which the authors suggest a complex role for Ca2+, since chelation of either intra- and extracellular Ca2+ as well as elevating cytosolic Ca2+ suppressed autophagy [33]. Since then the mechanism by which Ca2+ controls the autophagy remains controversial [18]. Previous studies have shown an inhibitory action of Ca2+ on autophagy [11, 31] while many recent studies showed a positive role of Ca2+ that activate autophagy [18, 34-36]. The majority of studies showing Ca2+ permeable channels as autophagy regulators are focused on the inositol trisphosphate receptor (IP3R) which is the main intracellular Ca2+ release channel [37].
1.3 IP3R as autophagic regulator
The stimulatory role of IP3R on starvation-induced autophagy was initially studied by Decuypere et al., 2011 where they showed that the Ca2+ chelator BAPTA-AM as well as the IP3R inhibitor xestospongin B abolished the starvation induced increase in the autophagy marker LC3 lipidation and GFP-LC3-puncta formation. Furthermore, starvation leads to IP3R sensitization through increased Beclin1 binding to the IP3R and a consequent decrease in Bcl2-Beclin1 interaction [18, 38, 39]. The autophagy protein Beclin1 promotes autophagosome formation by interacting with class III PI3-K, p150myristoylated kinase, when it is not bound with Bcl2 [40]. Moreover, autophagy might be inhibited by overexpression of Bcl2 and increased proliferation is observed upon Bcl2 overexpression. Consistent with these findings, Wang et al., 2008 reported that cadmium induces autophagy through elevation of cytosolic Ca2+ via the IP3R and subsequent extracellular signal-regulated kinase (ERK) activation [41]. In contrast, IP3R inhibitor xestospongin or IP3R knockdown also induced autophagy in HeLa cells [42, 43]. Importantly, triple IP3R-deficient DT40 cells demonstrated higher basal autophagy levels as compared to wild-type [44, 45]. Remarkably, the expression of functional IP3R3, but not Ca2+ impermeable mutant IP3R3D2550A, was able to rescue elevated autophagy in these cells [44]. The authors proposed the mechanism in which constitutive IP3R mediated Ca2+ release is taken up by mitochondria and this uptake is fundamentally required to maintain mitochondrial bioenergetics and ATP production in resting cells thereby suppressing autophagy [44]. Together, these findings indicate a bimodal role for Ca2+ release channels in the induction of autophagy, where basal autophagy is independent of IP3R, whereas induced autophagy may require Ca2+ release from IP3R.
To further complicate the role of IP3R, studies by Sarkar et al., 2005 showed decreases in IP3 levels by lithium induced mTOR independent autophagy [46]. Interestingly, mTOR is a regulator of autophagy, but also functions as ATP sensor. Further, TORC1 (protein involved in mTOR) has been shown to maintain macroautophagy at low basal levels [47], whereas, TORC1 inhibition by nutrient starvation or rapamycin (a macrolide that scavenges mTOR through FKBP12) unleashes massive macroautophagy [45] that might explain some of these discrepancies. In contrast, inhibition of inositol monophosphatase, which also decreases IP3 levels, showed an increase in autophagy [46]. Collectively these reports suggest a complex role for IP3R, since both stimulatory as well as inhibitory functions for IP3R toward autophagy has been reported [11, 18, 19]. There could be several reasons for these different results; first, different cells and their growth conditions might regulate these processes differently. Second, phosphorylation of Beclin1 has also been shown to promote its dissociation from Bcl-xL that could explain the differential effects of IP3R on cell survival after induction of autophagy. Third, Ca2+ has also been shown to regulate phosphorylation of many proteins including Bcl2 and Bcl-xL that might contribute to a different outcome. Fourth, evidence also suggests that Bcl2 inhibits autophagy by lowering ER Ca2+ levels that is independent of IP3R. Fifth, multiplicity and cross reactivity among these pathways can also explains the lack of simple generalization of the role of individual components, such as Ca2+ signaling and finally, release of ER Ca2+ also activates various Ca2+ entry channels that could further stimulate or inhibit autophagy (Figure 3).
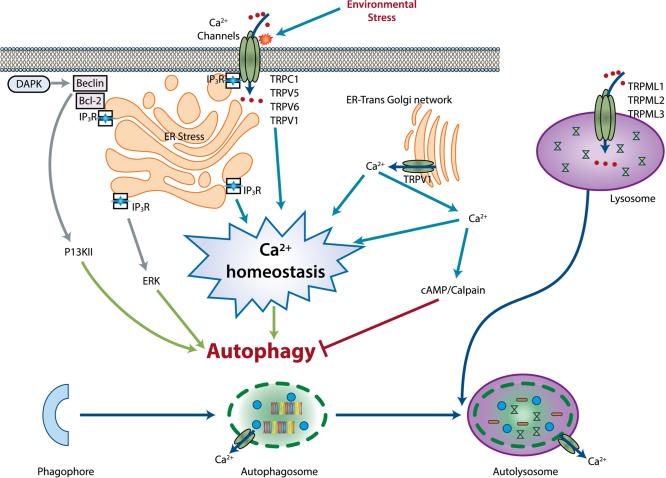
Figure 3. Role of intracellular Ca2+ and Ca2+ permeable channels in autophagy.
Schematic diagram showing intracellular Ca2+ and Ca2+ permeable channels in the control of autophagy. Stimulation of Ca2+ permeable channels by various environmental stresses like nutrient depression and serum starvation activates both excited and non-excited Ca2+ channels in the neuronal and non-neuronal cells. Stimulation of the channels also results in release of the store Ca2+ from the ER and Golgi bodies. These results in ER stress and disturbed Ca2+ homeostasis in the cells; which via various Ca2+ regulated proteins like ERK, calpains, cAMP regulates the autophagy processes.
2. TRP channels and Ca2+ signaling
The transient receptor potential (TRP) channel superfamily is one of the largest families of cation channels [48]. The TRP family is divided into subfamilies which are TRPC (canonical), TRPM (melastatin), TRPP (polycystin), TRPV (vanilloid), TRPML (mucolipin), TRPA (ankyrin) and TRPN (NOMPC-like); the latter is found only in invertebrates and fish [49] (Figure 1B). Importantly, all members of TRP family are moderately conserved and share significant homology among them [8, 50, 51]. The phylogenetic tree of the mammalian (human) TRP channel superfamily and the channels shown to involve in autophagy and ER stress is shown in figure 1B. Currently there are more than 21 TRP gene identified in various animals. TRP channels are involved in regulating various cellular functions, ranging from pure sensory function to molecular regulation, hence they serve as gatekeepers for transcellular transport of sodium and calcium ions [48, 52, 53]. The TRP protein in general is a six putative transmembrane protein with a pore forming reentrant loop between S5 and S6 [55]. Most of the TRPs, especially TRPCs, function as homotetramers, though the formation of heteromultimeric channels has also been reported [48]. TRPC is a subfamily of transient receptor potential channels that have the highest degree of homology to the first discovered Drosophila photoreceptors’ TRP channels [54].
2.1 TRP channels as regulators for autophagy
A relationship between IP3R and autophagy has been discussed above; however there are other Ca2+ permeable channels that are shown to be involved in the regulation of autophagy. In addition, release of ER Ca2+ (via the IP3R) activates plasma membrane Ca2+ channels that could further enhance these processes. Among them are the transient receptor potential (TRP) channel mainly transient receptor potential mucolipin-1 (TRPML1), also known as mucolipin-1, TRPML3, transient receptor potential vanilloid channel 1 (TRPV1), transient receptor potential canonical 1 (TRPC1) and recently the transient receptor potential melastatin 7 (TRPM7) [55-60] (Figure 1B and 3).
2.2 TRPML channels and autophagy
Several studies have proposed TRPML1 as an autophagic regulator [20]. It has been shown to be accompanied by the impairment of lysosomal pH, accumulation of autophagosomes and abnormal mitochondria, accumulation and aggregation of p62, and ubiquitin proteins, all of which are proposed to be a part of defective autophagy [61-64]. Vergarajauregui et al., 2008 demonstrated that TRPML1 is necessary for efficient fusion of both autophagosomes and late endosomes with lysosomes. They also showed that accumulation of autophagosomes in TRPML1-deficient fibroblasts obtained from mucolipidosis type IV patients was due to increased Beclin-1 dependent autophagosome formation and delayed fusion of autophagosomes with late endosomes/lysosomes [63]. In another study they showed that CMA is attenuated in mucolipidosis type IV fibroblasts and TRPML1 directly interacts with Hsp70 and Hsp40, members of molecular chaperone complex required for CMA. They postulated that this interaction may be required for intra lysosomal Hsp70 which facilitates the translocation of CMA substrate proteins across the lysosomal membrane [65]. Later in 2010, the same group investigated macroautophagy in neurons isolated from cerebellum of TRPML1−/− mouse embryos [66]. These cells showed higher levels of basal autophagy markers compared to wild-type ones. In addition, the autophagy marker LC3 clearance was affected in these cells, suggesting weakening of lysosomal function. Recent studies also showed that lysosomal Ca2+ signaling regulates autophagy through calcineurin and its substrate TFEB [26]. Lysosomal Ca2+ release through mucolipin 1 stimulates calcineurin which binds and dephosphorylates its substrate TFEB and thereby promoting its nuclear translocation to initiate autophagy [26].
In addition to TRPML1, other members of mucolipin family, TRPML2 and TRPML3, are also involved in autophagy regulation [67, 68]. In contrast to TRPML1, TRPML3 exhibits more limited tissue distribution and is mostly localized to early as well as late endosomes/lysosomes and less to the PM [59, 69]. Recent studies by Kim et al., 2009 showed that overexpression of TRPML3 leads to increased autophagy in HeLa cells and that TRPML3 channels are engaged to autophagosomes upon induction of autophagy [70]. Furthermore, expression of dominant negative mutant TRPML3 (D458K) or knockdown of endogenous TRPML3 by siRNA reduces autophagy. Thus, they proposed that TRPML3 provides the Ca2+ that is required for fusion and fission events in autophagy [67]. Hetero-multimerization of TRPML channels also have been shown to have a role in autophagy [69]. A recent study showed that TRPML3 interacts with mammalian ATG8 homologue GATE16 to regulate autophagy, thereby suggesting a vital role of TRPML3 in autophagasome maturation through the interaction with GATE16 [71].
2.3 TRPV channels modulate autophagy
Other TRP channels, mainly the transient receptor potential vanilloid channel 1 (TRPV1), have been proposed to regulate autophagy in thymocytes through reactive oxygen species (ROS)-regulated AMPK and Atg4C pathways [57]. It has been shown that capsaicin, an activator of TRPV1, persuades Beclin-1 dependent accumulation of LC3-II protein. This LC3-II accumulation can be antagonized by capsazepine, a blocker of TRPV1, and compound C, an AMPK inhibitor, suggesting AMPK involvement. Later the same group showed that the loss of TRPV2 homeostatic controls the proliferation and tumor progression in glioblastomas [72]. The study also showed that prostate cancer cells had a Ca2+ dependent activation of TRPV2 which increased the invasiveness of tumor cells. Capsaicin induced autophagy is Ca2+ dependent, as co-treatment with EDTA markedly reduced LC3-II accumulation. Moreover, capsaicin induces accumulation of ATG4C and triggers its oxidation in a ROS-dependent manner, thus regulating LC3 lipidation levels [57]. However, capsaicin has also showed to have TRPV1-independent effects, such as inhibition of voltage-gated Ca2+ channels [73]. Additionally, upon prolonged exposure to capsaicin, TRPV1 desensitization occurs and its activity decreases [74]. Thus, additional experiments using more specific agonists and antagonists as well as siRNA knockdown are needed to confirm the role of TRPV1 in autophagy regulation. It would be interesting as well to compare the effect of capsaicin on autophagy in TRPV1-expressing and TRPV1-null cells. Recent studies showed that the degradation of TRPV1 in HeLa cells are mediated by autophagy and that this pathway can be amended by cortisol [58].TRPV6 channel translocate to the PM through Orai1 mediated mechanism and control cancer cell survival and thereby could also potentially modulate autophagy [75]. TRPML3 and TRPV5 associate to form a novel heteromeric ion channel in dermal melanocytes and possibly involved in TRPML3 mediated regulation of autophagy [76]
2.4 TRPC channels as autophagic regulators
Recent studies from our lab showed the transient potential canonical channel 1 (TRPC1) as a key regulator in hypoxia and nutrient depletion dependent autophagy [60]. We demonstrated that an increase in intracellular Ca2+ via TRPC1 regulates autophagy, thereby preventing cell death in two morphologically distinct cells lines. Silencing of TRPC1 or inhibition of autophagy by 3-Methyladenine, attenuated hypoxia-induced increase in intracellular Ca2+ influx, decreased autophagy, and increased cell death [60]. Kim et al also showed that TRPC3 depletion reduced SOC and the severity of acute pancreatitis in mice. The authors also demostrated that all stressors that increase SOC activity and induce pancreatitis activate the ER stress response and induced autophagy in pancreatic acini. Deletion of TRPC3 reduced the rate of PERK phosphorylation and also reduced the rate of activation of autophagy in response to supramaximal CCK8 and to bile acids. This reduced the Ca2+ influx in Trpc3−/− cells and protected them by reducing ER stress and autophagy [77]. However, knockdown of TRPC3 did not affect the hypoxia induce autophagy in our studies [60].
2.5 TRPM7 channels as a regulator of basal autophagy
Recent studies by Chung's lab showed the role of TRPM7 channel in regulation of basal autophagy. Knockdown of endogenous TRPM7 channel in SH-SY5Y neuronal cells resulted in decreased basal autophagy. Further, when TRPM7 channels were expressed in HEK293 cells in a nutrient rich condition, the LC3-II level expression increased indicating a significant role of TRPM7 channels in basal autophagy [56].
2.6 TRPM2 channels and autophagy
TRPM2 ion channel has been shown to involve in H2O2-induced autophagy [20, 78, 79]. TRPM2 works both as ion channel and an enzyme [80] and has been shown to be activated and regulated by a variety of stimuli like H2O2 and cytosolic Ca2+. TRPM2-mediated Ca2+ regulates the interplay between ROS and autophagy [79]. ROS activates TRPM2 via another calcium mobilizing agent ADR-ribose (ADPR) inhibits early autophagy. TRPM2 also activates the calmodulin-dependent protein kinase II (CaMKII) to phosphorylate Ser295 on Beclin1 [78, 79].
2.7 Autophagy via voltage gated channels
Some ion channels, which do not belong to the family of TRP channels, are also proposed to regulate autophagy. Williams et al. found that L-type Ca2+ channels antagonists, namely verapamil, loperamide, nimodipine, nitrendipine and amiodarone, induce mTOR-independent autophagy [81]. The study also demonstrated that elevated cytosolic Ca2+, presumably due to activity of L-type Ca2+ channels on the PM, can activate calpains, which cleave and activate the α-subunit of heterotrimeric G proteins Gsα. Gsα activation, in turn, increases adenylyl cyclase activity leading to increase in cAMP levels which enhance the IP3 production. Hence, elevated intracellular cAMP levels negatively regulate autophagy by promoting IP3 production via cAMP-Epac-Rap2B-PLC-ε pathway. In addition, it has been well established that Ca2+ entry through these channels activate calmodulin, which in turn activates calmodulin-dependent serine/threonine kinases. These kinases play an important role in autophagy by facilitating the formation of autophagosomes and stimulating vesicular traffic [82]
3. Interplay between ER stress and autophagy
Autophagy is induced by protein aggregation and oxidative stress, which is dependent on the production of reactive oxygen species (ROS). Thus, ER stress and autophagy are often activated in parallel, share signaling pathways (particularly the Ca2+ signaling machinery), and team up to remove toxic byproducts of protein misfolding. Morphological changes of cells under ER stress were observed using electron microscopy and showed that autophagosome formation is accelerated when cells are under ER stress. Furthermore, the disturbance of autophagy rendered cells vulnerable to ER stress, suggesting that autophagy plays important roles in cell survival after ER stress [17]. The molecular mechanisms that link ER stress to autophagy may vary and various groups have proposed different hypotheses by which these two pathways cross-talk [30, 83] (Figure 3 and 4). For instance, Ca2+ mobilizing agents such as thapsigargin (an irreversible inhibitor of the ER Ca2+ ATPase), ionomycin, and ATP (via purinergic receptors) reportedly inhibit the activity of mTOR, a negative regulator of autophagy, and induce colossal accumulation of autophagosomes in a Beclin1- and Atg7-dependent manner [84]. In this regard, it has been proposed that Ca2+ release from the ER stimulates a CaMKKβ/AMPK-dependent pathway leading to the phosphorylation of the tumor suppressor tuberous sclerosis proteins 1/2 (TSC1/TSC2) complex and the downstream repression of mammalian target of rapamycin (mTOR) with the subsequent induction of autophagy [32]. Furthermore, Ogata et al. demonstrated that ER stress-induced autophagy is regulated by IRE1α interaction with TRAF2 to regulate jun amino-terminal kinases (JNK) activation [17]. Recent studies showed that JNK-mediated phosphorylation of Bcl2 caused its release of Beclin1, thereby allowing autophagy to proceed [85]. In addition, ER stress also lead to an increase in the expression of transcription factor CHOP, which may also contribute towards autophagy as it is known to down regulate Bcl2 [86] and activate the transcription of ATG5 [87].
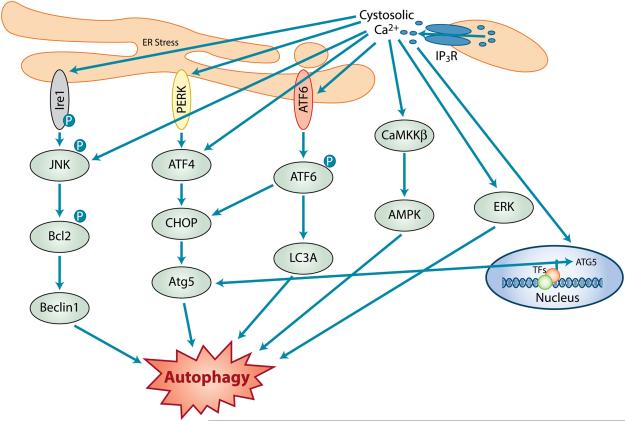
Figure 4. Ca2+, ER stress and autophagy.
Schematic diagram showing the three known signaling pathways which implicates ER stress- induced autophagy. Three of the UPR responses include the Ire1/JNK/Bcl2/Beclin1 PERK/ATF4/CHOP/Atg5 and ATF6/LC3A which have been implicated in signaling of ER stress-induced autophagy. The ER stress- associated increase intracellular Ca2+ is mainly through the activation of IP3R which activates the CAMKK-β and ERK pathways which also leads to autophagy.
3.1 ER stress
ER stress is a condition that disrupts the redox balance and luminal Ca2+ homeostasis, thereby resulting in the accumulation of unfolded/misfolded proteins. Activation of ER stress is an evolutionary conserved adaptive response named unfolded protein response (UPR), which is initiated by three major signal transducers of the ER membrane: the protein kinase-like ER kinase (PERK), the inositol requiring enzyme (IRE1) and the activating transcription factor 6 (ATF-6) (Figure 4). ER stress leads to the activation of two protein degradation pathways. First, the ubiquitin-proteasome pathway that is via the endoplasmicreticulum-associated protein degradation (ERAD) protein. Second, UPR and lysosome-mediated protein degradation pathway that is via the autophagy pathway [83]. ERAD involves retro-translocation of unfolded ER proteins to the cytosol where they are ubiquitinated and degraded by the proteasome. When the buildup of misfolded or unfolded proteins exceeds the ER capacity, autophagy can be induced as a secondary response to UPR and degrade accumulated proteins and thus alleviate ER stress [30]. Although ER stress-induced cell death can proceed in both Ca2+-independent and Ca2+-dependent ways, here we have only focused on Ca2+-dependent pathways.
3.2 Mechanism of ER stress induced autophagy
In autophagy, the formation of the autophagosome membrane requires the sequential action of numerous proteins involved in vesicle nucleation, fusion, elongation, with lysosomes, and final degradation of engulfed substrates. The first regulatory process involves attenuation of the mTOR Ser/Thr kinase, which blocks autophagy by phosphorylation of Atg13, and the dissociation of this protein forms a complex formed by Atg1 (a protein kinase) and Atg17. The primary step of vesicle nucleation is the activation of Vps34, a class III phosphatidylinositol 3-kinase, which associates with a complex formed by Beclin-1, UV irradiation resistance-associated tumor suppressor gene (UVRAG), and the kinase Vps15/p150. Beclin1 can also interrelate with the anti-apoptotic protein Bcl2 at the ER, with Bcl2 inhibiting starvation-induced autophagy [85]. The next phase of elongation involves two ubiquitin-like conjugation steps. First, the proteins Atg12 and Atg5 are covalently conjugated together with the cooperation of Atg7 (E1-like) and Atg10 (E2-like). Second, conjugation of Atg8 with phosphatidylethanolamine (PE) in the membranes of autophagic vesicles occurs following its proteolytic cleavage by Atg4, a cysteine protease [88]. The subsequent recruitment of Atg8 and other autophagy-related proteins is believed to trigger vesicle expansion in a concerted manner, presumably by providing the driving force for membrane curvature [89]. The transient conjugation of Atg8 to the membrane lipid PE is essential for phagophore expansion as its mutation leads to defects in autophagosome formation [90]. It is distributed symmetrically on both sides of the autophagosome and it is assumed that there is a quantitative correlation between the amount of Atg8 and the vesicle size [91]. After finishing vesicle expansion, the autophagosome is ready for fusion with the lysosome and Atg8 can either be released from the membrane for recycling or be degraded in the autolysosome. Ogata et al in 2006 studied neuronal cell survival after ER stress and revealed autophagy was induced after ER stress in a manner similar to the wild-type cells when UPR protein ATF6 was knocked down. This suggests that autophagy plays an important role in cell survival after ER stress [17].
3.3 ROS mediates autophagy induction in response to ER stress
Pérez-Martín et al, 2014 showed that in Chlamydomonas the accumulation of misfolded proteins in the ER under tunicamycin or DTT treatment increased the expression of the ATG8 gene [92], which is important for the induction of autophagy. Pronounced accumulation of the ATG8 protein and its modified forms were also detected in ER stressed cells. They also showed that the ATG8 induction and ER stress caused by DTT is attenuated by exogenous glutathione (GSH). Glutathione plays a vital role in inhibiting reactive oxygen species (ROS) and in the formation of disulfide bonds in the ER associated with oxidative-induced protein folding that are linked to the generation of H2O2 by the activity of the ERO1 oxidoreductase [93, 94]. Hence it could be suggested that downregulation of autophagy might be due to the partial subdual of ER stress or to GSH-dependent ROS scavenging. Like tunicamycin treatment, SERCA inhibitor, thapsigargin also led to ER stress and autophagy activation in Chlamydomonas [92]. Suggesting a significant role of store operated Ca2+ entry in ROS and ER stress induced autophagy in Chlamydomonas. Moreover, recently it was shown that the proautophagic and antioxidant functions of the ER resident protein kinase, PERK that operate during normal mammary acinus development are challenged in breast tumor cells for them to survive oxidative stress and resist anoikis [95]. Importantly, PERK also regulated cell redox homeostasis via buffering ROS accumulation. Similarly, recent studies by Shen et al in human choriocarcinoma showed that the switch from ER-stress induced apoptosis to autophagy is via ROS and is mediated through the activation of JNK/p62 pathway [96]. Redox regulation of protein by moderate levels of ROS is also observed in autophagy. Accumulation of ROS induces autophagy, which in turn serves to attenuate the ROS levels have been also shown [97]. Many studies hypothesize that ROS are crucial for autophagy execution as treatment with antioxidants reverts the process [98]. Ca2+ play a vital role in ROS-mediated autophagy process and extensive literature in regulation of autophagy by ROS and role of calcium in ROS is illustrated in recent articles [19, 99]. Thus, it could be anticipated that TRP channels that are main regulators for calcium entry will contribute to these processes; however more research is needed to verify their role in various conditions.
4. ER stress and TRP channels
As we've discussed, disruption of Ca2+ homeostasis in the ER is considered an important trigger of ER stress. Members of the TRP family have been shown to mediate cellular Ca2+ homeostasis and initiate ER stress through different mechanisms. For example the channels TRPC1[100, 101], TRPV1 [102-109], and TRPC6[110] are expressed at both the ER membrane as well as in the plasma membrane and have been linked to ER Ca2+ homeostasis. Further, the channels TRPC3 and TRPC6 have each been identified as having a role in ER stress-induced apoptosis [111, 112]. Together it can be acknowledged that TRP channels have a role in maintaining ER Ca2+ homeostasis and disruption of channel function leads to ER stress.
4.1 TRPC channels as inhibitors of ER stress
Transient receptor potential channel 1 (TRPC1) plays a vital role in maintaining ER Ca2+ homeostasis and reduction in its function leads to prolonged activation of the UPR pathway and impairs AKT activation, which subsequently leads to neurodegeneration [113]. Our lab has uncovered a direct correlation between TRPC1 and ER stress in dopaminergic neurons of the substantia nigra, where endogenous store-operated Ca2+ entry (SOCE), which is critical for maintaining ER Ca2+ levels, is dependent on TRPC1 activity [101]. In this study, a neurotoxin-induced mouse model for Parkinson's disease showed decreased TRPC1 expression, TRPC1 interaction with the SOCE modulator stromal interaction molecule 1 (STIM1), and Ca2+ entry into the cells. However, the overexpression of functional TRPC1 protected against neurotoxin-induced ER stress and UPR by restoring AKT/mTOR signaling and increasing DA neuron survival. Although the role of autophagy has not been directly assessed in these instances, it is sensible to propose that activation of cell death pathways due to prolonged ER stress sets off a protective response mediated at least partly by autophagy as shown above.
Another TRP channel to play a role in ER stress is TRPC3. Within mouse epithelial cells from pancreatic and parotid acini, the loss of TRPC3 function ameliorates ER stressed induced UPR via PERK signaling [114]. ER stress can cause the activation of Ca2+ /calmodulin-dependent protein kinase II (CAMKII) and work done in HCAECs has shown Ca2+ influx by TRPC3 is required for the activation of CAMKII within UPR and the eventual ER-stress induced apoptosis [111, 115, 116]. It has also been shown that canonical transient receptor potential-6 (TRPC6) is expressed in the ER membrane blood platelets [110] and in podocytes and the channel can be activated by albumin [112]. Overloading the cell with albumin causes an excess of Ca2+ entry resulting in the expression ER stress protein GFP78 and eventual apoptosis. Knocking down TRPC6 abolishes the ER stress and subsequent apoptosis indicating a clear linkage between TRPC6 and ER stress induced apoptosis [112].
4.2 TRPV channels and ER stress
The channel Transient receptor potential vanilloid 1 (TRPV1) has a strong correlation to ER stress due to its expression in the ER membrane. It has been shown in human lung cells, that agonists for this subpopulation of TRPV1 in the ER disrupts ER Ca2+ homeostasis and activates EIF2αK3-dependent ER stress responses [117]. In the same study, the use of a TRPV1 agonist caused a Ca2+ release from the ER along with subsequent increased expression of stress-response genes GADD153, GADD45α, ATF3, CCNG2, and BiP/GRP78 mRNA and a decrease in CCND indicating stress to the ER similar to that of ER stress inducing-agents thapsigargin and DTT [14]. Further work indicated inflammation produced endogenous TRPV1 agonists activating TRPV1 in lung cells, thus causing ER stress, GADD153 expression, and lung injury [118]. Within dorsal root ganglion neurons it was found that these ER bound TRPV1 channels have a low sensitivity for agonists such as capsaicin as compared to plasma membrane bound TRPV1 channels, possibly indicating a critical safety mechanism to protect the neurons from Ca2+ depletion of ER, leading to ER stress, unfolding protein response, and cell death [104].
5. Conclusion and future directions
The cellular “self-eating” phenomenon of autophagy and ER stress and their cross-talk mechanisms are involved in the maintenance of cellular energetics and cell survival. Over the last few years Ca2+-permeable ion channels have emerged as an important regulator for these two vital processes. The effect of such regulation mostly depends on the Ca2+ signals in a spatially restricted subcellular domain that is achieved by many proteins and ion channels as discussed in this review; however more research is needed to fully dissect these intricate relationships. The interactions among the ER, mitochondria and lysosomes are crucial for cell survival, but a comprehensive signaling pathway for activation of the autophagy induced by ER stress awaits further analysis as the role of Ca2+ signaling is still controversial. Importantly, most of the studies that have shown this important relationship are performed in cell culture models and future studies using animal models will be necessary to resolve some of these issues. Nevertheless, both autophagy and ER stress have been associated in certain human diseases, such as Cancer progression (loss of autophagy), Parkinson's disease, Alzheimer, ALS, and Huntington's disease, and exploration of the novel signaling pathways relevant to ER stress and autophagy could lead to the development of new therapeutic strategies for these diseases. Deregulation of Ca2+ homeostasis, ER stress and autophagy also impairs mitochondrial function, leading to a decrease in ATP production that can make these cells vulnerable to insults. Thus, further studies are still needed to understand the variety of mechanisms, by which Ca2+ channels can influence these processes and could also have a broad impact on understanding and developing potential clinical drugs against these diseases.
Acknowledgements
We thank the grant support from the NIH (DE017102, DE024300-01A1) awarded to B.B.S, and the assistance of John Swift, School of Medicine and Health Sciences in making the figures.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Prevarskaya N, Skryma R, Shuba Y. Calcium in tumour metastasis: new roles for known actors. Nat. Rev. Cancer. 2011;11:609–618. doi: 10.1038/nrc3105. [DOI] [PubMed] [Google Scholar]
- 2.Ouyang D-Y, Xu L-H, He X-H, Zhang Y-T, Zeng L-H, Cai J-Y, Ren S. Autophagy is differentially induced in prostate cancer LNCaP, DU145 and PC-3 cells via distinct splicing profiles of ATG5. Autophagy. 2013;9:20–32. doi: 10.4161/auto.22397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vasko VV, Saji M. Molecular mechanisms involved in differentiated thyroid cancer invasion and metastasis. Curr Opin Oncol. 2007;19:11–17. doi: 10.1097/CCO.0b013e328011ab86. [DOI] [PubMed] [Google Scholar]
- 4.Bödding M. TRP proteins and cancer. Cell. Signal. 2007;19:617–624. doi: 10.1016/j.cellsig.2006.08.012. [DOI] [PubMed] [Google Scholar]
- 5.Ong HL, de Souza LB, Cheng KT, Ambudkar IS. Physiological functions and regulation of TRPC channels. Handb Exp Pharmacol. 2014;223:1005–1034. doi: 10.1007/978-3-319-05161-1_12. [DOI] [PubMed] [Google Scholar]
- 6.Yamakage M, Namiki A. Calcium channels--basic aspects of their structure, function and gene encoding; anesthetic action on the channels--a review. Can J Anaesth. 2002;49:151–164. doi: 10.1007/BF03020488. [DOI] [PubMed] [Google Scholar]
- 7.Berridge MJ, Bootman MD, Roderick HL. Calcium signalling: dynamics, homeostasis and remodelling. Nat. Rev. Mol. Cell Biol. 2003;4:517–529. doi: 10.1038/nrm1155. [DOI] [PubMed] [Google Scholar]
- 8.Abramowitz J, Birnbaumer L. Physiology and pathophysiology of canonical transient receptor potential channels. FASEB J. 2009;23:297–328. doi: 10.1096/fj.08-119495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tornquist K, Sukumaran P, Kemppainen K, Lof C, Viitanen T. Canonical transient receptor potential channel 2 (TRPC2): old name-new games. Importance in regulating of rat thyroid cell physiology. Pflugers Archiv : European journal of physiology. 2014;466:2025–2034. doi: 10.1007/s00424-014-1509-z. [DOI] [PubMed] [Google Scholar]
- 10.Cole K, Kohn E. Calcium-mediated signal transduction: biology, biochemistry, and therapy. Cancer Metastasis Rev. 1994;13:31–44. doi: 10.1007/BF00690417. [DOI] [PubMed] [Google Scholar]
- 11.East DA, Campanella M. Ca2+ in quality control: an unresolved riddle critical to autophagy and mitophagy. Autophagy. 2013;9:1710–1719. doi: 10.4161/auto.25367. [DOI] [PubMed] [Google Scholar]
- 12.Lof C, Viitanen T, Sukumaran P, Tornquist K. TRPC2: of mice but not men. Advances in experimental medicine and biology. 2011;704:125–134. doi: 10.1007/978-94-007-0265-3_6. [DOI] [PubMed] [Google Scholar]
- 13.Borle AB. Control, Modulation, and regulation of cell calcium. Rev. Physiol. Biochem. Pharmacol. 1981;90:13–153. doi: 10.1007/BFb0034078. [DOI] [PubMed] [Google Scholar]
- 14.Schroder M, Kaufman RJ. ER stress and the unfolded protein response. Mutation research. 2005;569:29–63. doi: 10.1016/j.mrfmmm.2004.06.056. [DOI] [PubMed] [Google Scholar]
- 15.Woehlbier U, Hetz C. Modulating stress responses by the UPRosome: a matter of life and death. Trends Biochem Sci. 2011;36:329–337. doi: 10.1016/j.tibs.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 16.Verfaillie T, Rubio N, Garg AD, Bultynck G, Rizzuto R, Decuypere JP, Piette J, Linehan C, Gupta S, Samali A, Agostinis P. PERK is required at the ER-mitochondrial contact sites to convey apoptosis after ROS-based ER stress. Cell Death Differ. 2012;19:1880–1891. doi: 10.1038/cdd.2012.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ogata M, Hino S, Saito A, Morikawa K, Kondo S, Kanemoto S, Murakami T, Taniguchi M, Tanii I, Yoshinaga K, Shiosaka S, Hammarback JA, Urano F, Imaizumi K. Autophagy is activated for cell survival after endoplasmic reticulum stress. Mol Cell Biol. 2006;26:9220–9231. doi: 10.1128/MCB.01453-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Decuypere JP, Bultynck G, Parys JB. A dual role for Ca(2+) in autophagy regulation. Cell Calcium. 2011;50:242–250. doi: 10.1016/j.ceca.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 19.Kania E, Pajak B, Orzechowski A. Calcium homeostasis and ER stress in control of autophagy in cancer cells. BioMed research international. 2015:352794–352804. doi: 10.1155/2015/352794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kondratskyi A, Yassine M, Kondratska K, Skryma R, Slomianny C, Prevarskaya N. Calcium-permeable ion channels in control of autophagy and cancer. Frontiers in physiology. 2013;4:272–284. doi: 10.3389/fphys.2013.00272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Murrow L, Debnath J. Autophagy as a stress-response and quality-control mechanism: implications for cell injury and human disease. Annual review of pathology. 2013;8:105–137. doi: 10.1146/annurev-pathol-020712-163918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ravikumar B, Sarkar S, Davies JE, Futter M, Garcia-Arencibia M, Green-Thompson ZW, Jimenez-Sanchez M, Korolchuk VI, Lichtenberg M, Luo S, Massey DC, Menzies FM, Moreau K, Narayanan U, Renna M, Siddiqi FH, Underwood BR, Winslow AR, Rubinsztein DC. Regulation of mammalian autophagy in physiology and pathophysiology. Physiol Rev. 2010;90:1383–1435. doi: 10.1152/physrev.00030.2009. [DOI] [PubMed] [Google Scholar]
- 23.Kato M, Ospelt C, Gay RE, Gay S, Klein K. Dual role of autophagy in stress-induced cell death in rheumatoid arthritis synovial fibroblasts. Arthritis & rheumatology. 2014;66:40–48. doi: 10.1002/art.38190. [DOI] [PubMed] [Google Scholar]
- 24.Zhang Y, Calderwood SK. Autophagy, protein aggregation and hyperthermia: a mini-review. International journal of hyperthermia : the official journal of European Society for Hyperthermic Oncology, North American Hyperthermia Group. 2011;27:409–414. doi: 10.3109/02656736.2011.552087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ouyang L, Shi Z, Zhao S, Wang FT, Zhou TT, Liu B, Bao JK. Programmed cell death pathways in cancer: a review of apoptosis, autophagy and programmed necrosis. Cell proliferation. 2012;45:487–498. doi: 10.1111/j.1365-2184.2012.00845.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Medina DL, Di Paola S, Peluso I, Armani A, De Stefani D, Venditti R, Montefusco S, Scotto-Rosato A, Prezioso C, Forrester A, Settembre C, Wang W, Gao Q, Xu H, Sandri M, Rizzuto R, De Matteis MA, Ballabio A. Lysosomal calcium signalling regulates autophagy through calcineurin and TFEB. Nat Cell Biol. 2015;17:288–299. doi: 10.1038/ncb3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Medina DL, Ballabio A. Lysosomal calcium regulates autophagy. Autophagy. 2015 doi: 10.1080/15548627.2015.1047130. 0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huang Z, Zhou L, Chen Z, Nice EC, Huang C. Stress Management by Autophagy: Implications for Chemoresistance. International journal of cancer. Journal international du cancer. 2016 doi: 10.1002/ijc.29990. [DOI] [PubMed] [Google Scholar]
- 29.Lin Z, Wu F, Lin S, Pan X, Jin L, Lu T, Shi L, Wang Y, Xu A, Li X. Adiponectin protects against acetaminophen-induced mitochondrial dysfunction and acute liver injury by promoting autophagy in mice. Journal of hepatology. 2014;61:825–831. doi: 10.1016/j.jhep.2014.05.033. [DOI] [PubMed] [Google Scholar]
- 30.Sovolyova N, Healy S, Samali A, Logue SE. Stressed to death - mechanisms of ER stress-induced cell death. Biol Chem. 2014;395:1–13. doi: 10.1515/hsz-2013-0174. [DOI] [PubMed] [Google Scholar]
- 31.Cardenas C, Miller RA, Smith I, Bui T, Molgo J, Muller M, Vais H, Cheung KH, Yang J, Parker I, Thompson CB, Birnbaum MJ, Hallows KR, Foskett JK. Essential regulation of cell bioenergetics by constitutive InsP3 receptor Ca2+ transfer to mitochondria. Cell. 2010;142:270–283. doi: 10.1016/j.cell.2010.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hoyer-Hansen M, Bastholm L, Szyniarowski P, Campanella M, Szabadkai G, Farkas T, Bianchi K, Fehrenbacher N, Elling F, Rizzuto R, Mathiasen IS, Jaattela M. Control of macroautophagy by calcium, calmodulin-dependent kinase kinase-beta, and Bcl-2. Mol Cell. 2007;25:193–205. doi: 10.1016/j.molcel.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 33.Gordon JN, Shu WP, Schlussel RN, Droller MJ, Liu BC. Altered extracellular matrices influence cellular processes and nuclear matrix organizations of overlying human bladder urothelial cells. Cancer Res. 1993;53:4971–4977. [PubMed] [Google Scholar]
- 34.Su J, Zhou L, Kong X, Yang X, Xiang X, Zhang Y, Li X, Sun L. Endoplasmic reticulum is at the crossroads of autophagy, inflammation, and apoptosis signaling pathways and participates in the pathogenesis of diabetes mellitus. Journal of diabetes research. 2013;2013:193461. doi: 10.1155/2013/193461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gastaldello A, Callaghan H, Gami P, Campanella M. Ca 2+ -dependent autophagy is enhanced by the pharmacological agent PK11195. Autophagy. 2010;6:607–613. doi: 10.4161/auto.6.5.11964. [DOI] [PubMed] [Google Scholar]
- 36.Smaili SS, Pereira GJS, Costa MM, Rocha KK, Rodrigues L, do Carmo LG, Hirata H, Hsu YT. The role of calcium stores in apoptosis and autophagy. Curr. Mol. Med. 2013;13:252–265. doi: 10.2174/156652413804810772. [DOI] [PubMed] [Google Scholar]
- 37.Parys JB, Decuypere JP, Bultynck G. Role of the inositol 1,4,5-trisphosphate receptor/Ca2+-release channel in autophagy. Cell communication and signaling : CCS. 2012;10:17. doi: 10.1186/1478-811X-10-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Decuypere JP, Welkenhuyzen K, Luyten T, Ponsaerts R, Dewaele M, Molgo J, Agostinis P, Missiaen L, De Smedt H, Parys JB, Bultynck G. Ins(1,4,5)P3 receptor-mediated Ca2+ signaling and autophagy induction are interrelated. Autophagy. 2011;7:1472–1489. doi: 10.4161/auto.7.12.17909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Decuypere JP, Monaco G, Bultynck G, Missiaen L, De Smedt H, Parys JB. The IP(3) receptor-mitochondria connection in apoptosis and autophagy. Biochim Biophys Acta. 2011;1813:1003–1013. doi: 10.1016/j.bbamcr.2010.11.023. [DOI] [PubMed] [Google Scholar]
- 40.Gao W, Ding WX, Stolz DB, Yin XM. Induction of macroautophagy by exogenously introduced calcium. Autophagy. 2008;4:754–761. doi: 10.4161/auto.6360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang SH, Shih YL, Ko WC, Wei YH, Shih CM. Cadmium-induced autophagy and apoptosis are mediated by a calcium signaling pathway. Cellular and molecular life sciences : CMLS. 2008;65:3640–3652. doi: 10.1007/s00018-008-8383-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Criollo A, Vicencio JM, Tasdemir E, Maiuri MC, Lavandero S, Kroemer G. The inositol trisphosphate receptor in the control of autophagy. Autophagy. 2007;3:350–353. doi: 10.4161/auto.4077. [DOI] [PubMed] [Google Scholar]
- 43.Criollo A, Maiuri MC, Tasdemir E, Vitale I, Fiebig AA, Andrews D, Molgo J, Diaz J, Lavandero S, Harper F, Pierron G, di Stefano D, Rizzuto R, Szabadkai G, Kroemer G. Regulation of autophagy by the inositol trisphosphate receptor. Cell Death Differ. 2007;14:1029–1039. doi: 10.1038/sj.cdd.4402099. [DOI] [PubMed] [Google Scholar]
- 44.Cardenas C, Juretic N, Bevilacqua JA, Garcia IE, Figueroa R, Hartley R, Taratuto AL, Gejman R, Riveros N, Molgo J, Jaimovich E. Abnormal distribution of inositol 1,4,5-trisphosphate receptors in human muscle can be related to altered calcium signals and gene expression in Duchenne dystrophy-derived cells. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2010;24:3210–3221. doi: 10.1096/fj.09-152017. [DOI] [PubMed] [Google Scholar]
- 45.Khan MT, Joseph SK. Role of inositol trisphosphate receptors in autophagy in DT40 cells. J Biol Chem. 2010;285:16912–16920. doi: 10.1074/jbc.M110.114207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sarkar S, Floto RA, Berger Z, Imarisio S, Cordenier A, Pasco M, Cook LJ, Rubinsztein DC. Lithium induces autophagy by inhibiting inositol monophosphatase. J Cell Biol. 2005;170:1101–1111. doi: 10.1083/jcb.200504035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dennis PB, Jaeschke A, Saitoh M, Fowler B, Kozma SC, Thomas G. Mammalian TOR: a homeostatic ATP sensor. Science. 2001;294:1102–1105. doi: 10.1126/science.1063518. [DOI] [PubMed] [Google Scholar]
- 48.Nilius B, Owsianik G. The transient receptor potential family of ion channels. Genome Biol. 2011;12 doi: 10.1186/gb-2011-12-3-218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nilius B. TRP channels in disease, Biochimica et Biophysica Acta (BBA) Molecular Basis of Disease. 2007;1772:805–812. doi: 10.1016/j.bbadis.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 50.Pan Z, Yang H, Reinach PS. Transient receptor potential (TRP) gene superfamily encoding cation channels. Human genomics. 2011;5:108–116. doi: 10.1186/1479-7364-5-2-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sukumaran P, Lof C, Kemppainen K, Kankaanpaa P, Pulli I, Nasman J, Viitanen T, Tornquist K. Canonical transient receptor potential channel 2 (TRPC2) as a major regulator of calcium homeostasis in rat thyroid FRTL-5 cells: importance of protein kinase C delta (PKCdelta) and stromal interaction molecule 2 (STIM2) J Biol Chem. 2012;287:44345–44360. doi: 10.1074/jbc.M112.374348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Löf C, Viitanen T, Sukumaran P, Törnquist K. TRPC2: of mice but not men. Adv. Exp. Med. Biol. 2011;704:125–134. doi: 10.1007/978-94-007-0265-3_6. [DOI] [PubMed] [Google Scholar]
- 53.Flockerzi V. An introduction on TRP channels. Handb Exp Pharmacol. 2007:1–19. doi: 10.1007/978-3-540-34891-7_1. [DOI] [PubMed] [Google Scholar]
- 54.Kiselyov K, van Rossum DB, Patterson RL. TRPC channels in pheromone sensing. Vitam. Horm. 2010;83:197–213. doi: 10.1016/S0083-6729(10)83008-0. [DOI] [PubMed] [Google Scholar]
- 55.Zeevi A, Lunz JG, 3rd, Shapiro R, Randhawa P, Mazariegos G, Webber S, Girnita A. Emerging role of donor-specific anti-human leukocyte antigen antibody determination for clinical management after solid organ transplantation. Human immunology. 2009;70:645–650. doi: 10.1016/j.humimm.2009.06.009. [DOI] [PubMed] [Google Scholar]
- 56.Oh HG, Chun YS, Park CS, Kim TW, Park MK, Chung S. Regulation of basal autophagy by transient receptor potential melastatin 7 (TRPM7) channel. Biochem Biophys Res Commun. 2015;463:7–12. doi: 10.1016/j.bbrc.2015.05.007. [DOI] [PubMed] [Google Scholar]
- 57.Farfariello V, Amantini C, Santoni G. Transient receptor potential vanilloid 1 activation induces autophagy in thymocytes through ROS-regulated AMPK and Atg4C pathways. Journal of leukocyte biology. 2012;92:421–431. doi: 10.1189/jlb.0312123. [DOI] [PubMed] [Google Scholar]
- 58.Ahn S, Park J, An I, Jung SJ, Hwang J. Transient receptor potential cation channel V1 (TRPV1) is degraded by starvation- and glucocorticoid-mediated autophagy. Mol Cells. 2014;37:257–263. doi: 10.14348/molcells.2014.2384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cheng X, Shen D, Samie M, Xu H. Mucolipins: Intracellular TRPML1-3 channels. FEBS Lett. 2010;584:2013–2021. doi: 10.1016/j.febslet.2009.12.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sukumaran P, Sun Y, Vyas M, Singh BB. TRPC1-mediated Ca(2)(+) entry is essential for the regulation of hypoxia and nutrient depletion-dependent autophagy. Cell death & disease. 2015;6:e1674. doi: 10.1038/cddis.2015.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jennings JJ, Jr., Zhu JH, Rbaibi Y, Luo X, Chu CT, Kiselyov K. Mitochondrial aberrations in mucolipidosis Type IV. J Biol Chem. 2006;281:39041–39050. doi: 10.1074/jbc.M607982200. [DOI] [PubMed] [Google Scholar]
- 62.Curcio-Morelli C, Charles FA, Micsenyi MC, Cao Y, Venugopal B, Browning MF, Dobrenis K, Cotman SL, Walkley SU, Slaugenhaupt SA. Macroautophagy is defective in mucolipin-1-deficient mouse neurons. Neurobiology of disease. 2010;40:370–377. doi: 10.1016/j.nbd.2010.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Vergarajauregui S, Connelly PS, Daniels MP, Puertollano R. Autophagic dysfunction in mucolipidosis type IV patients. Human molecular genetics. 2008;17:2723–2737. doi: 10.1093/hmg/ddn174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Soyombo AA, Tjon-Kon-Sang S, Rbaibi Y, Bashllari E, Bisceglia J, Muallem S, Kiselyov K. TRP-ML1 regulates lysosomal pH and acidic lysosomal lipid hydrolytic activity. J Biol Chem. 2006;281:7294–7301. doi: 10.1074/jbc.M508211200. [DOI] [PubMed] [Google Scholar]
- 65.Venugopal B, Mesires NT, Kennedy JC, Curcio-Morelli C, Laplante JM, Dice JF, Slaugenhaupt SA. Chaperone-mediated autophagy is defective in mucolipidosis type IV. J Cell Physiol. 2009;219:344–353. doi: 10.1002/jcp.21676. [DOI] [PubMed] [Google Scholar]
- 66.Curcio-Morelli C, Zhang P, Venugopal B, Charles FA, Browning MF, Cantiello HF, Slaugenhaupt SA. Functional multimerization of mucolipin channel proteins. J Cell Physiol. 2010;222:328–335. doi: 10.1002/jcp.21956. [DOI] [PubMed] [Google Scholar]
- 67.Kim HJ, Soyombo AA, Tjon-Kon-Sang S, So I, Muallem S. The Ca(2+) channel TRPML3 regulates membrane trafficking and autophagy. Traffic. 2009;10:1157–1167. doi: 10.1111/j.1600-0854.2009.00924.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cuajungco MP, Silva J, Habibi A, Valadez JA. The mucolipin-2 (TRPML2) ion channel: a tissue-specific protein crucial to normal cell function. Pflugers Archiv : European journal of physiology. 2016;468:177–192. doi: 10.1007/s00424-015-1732-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zeevi DA, Frumkin A, Offen-Glasner V, Kogot-Levin A, Bach G. A potentially dynamic lysosomal role for the endogenous TRPML proteins. The Journal of pathology. 2009;219:153–162. doi: 10.1002/path.2587. [DOI] [PubMed] [Google Scholar]
- 70.Kim SH, Park EJ, Lee CR, Chun JN, Cho NH, Kim IG, Lee S, Kim TW, Park HH, So I, Jeon JH. Geraniol induces cooperative interaction of apoptosis and autophagy to elicit cell death in PC-3 prostate cancer cells. International journal of oncology. 2012;40:1683–1690. doi: 10.3892/ijo.2011.1318. [DOI] [PubMed] [Google Scholar]
- 71.Choi S, Kim HJ. The Ca2+ channel TRPML3 specifically interacts with the mammalian ATG8 homologue GATE16 to regulate autophagy. Biochem Biophys Res Commun. 2014;443:56–61. doi: 10.1016/j.bbrc.2013.11.044. [DOI] [PubMed] [Google Scholar]
- 72.Liberati S, Morelli MB, Amantini C, Farfariello V, Santoni M, Conti A, Nabissi M, Cascinu S, Santoni G. Loss of TRPV2 Homeostatic Control of Cell Proliferation Drives Tumor Progression. Cells. 2014;3:112–128. doi: 10.3390/cells3010112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hagenacker T, Splettstoesser F, Greffrath W, Treede RD, Busselberg D. Capsaicin differentially modulates voltage-activated calcium channel currents in dorsal root ganglion neurones of rats. Brain Res. 2005;1062:74–85. doi: 10.1016/j.brainres.2005.09.033. [DOI] [PubMed] [Google Scholar]
- 74.Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- 75.Raphael M, Lehen'kyi V, Vandenberghe M, Beck B, Khalimonchyk S, Vanden Abeele F, Farsetti L, Germain E, Bokhobza A, Mihalache A, Gosset P, Romanin C, Clezardin P, Skryma R, Prevarskaya N. TRPV6 calcium channel translocates to the plasma membrane via Orai1-mediated mechanism and controls cancer cell survival. Proc Natl Acad Sci U S A. 2014;111:E3870–3879. doi: 10.1073/pnas.1413409111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Guo Z, Grimm C, Becker L, Ricci AJ, Heller S. A novel ion channel formed by interaction of TRPML3 with TRPV5. PLoS ONE. 2013;8:e58174. doi: 10.1371/journal.pone.0058174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kim MS, Hong JH, Li Q, Shin DM, Abramowitz J, Birnbaumer L, Muallem S. Deletion of TRPC3 in mice reduces store-operated Ca2+ influx and the severity of acute pancreatitis. Gastroenterology. 2009;137:1509–1517. doi: 10.1053/j.gastro.2009.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Miller BA, Hoffman NE, Merali S, Zhang XQ, Wang J, Rajan S, Shanmughapriya S, Gao E, Barrero CA, Mallilankaraman K, Song J, Gu T, Hirschler-Laszkiewicz I, Koch WJ, Feldman AM, Madesh M, Cheung JY. TRPM2 channels protect against cardiac ischemiareperfusion injury: role of mitochondria. J Biol Chem. 2014;289:7615–7629. doi: 10.1074/jbc.M113.533851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gao G, Wang W, Tadagavadi RK, Briley NE, Love MI, Miller BA, Reeves WB. TRPM2 mediates ischemic kidney injury and oxidant stress through RAC1. The Journal of clinical investigation. 2014;124:4989–5001. doi: 10.1172/JCI76042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sumoza-Toledo A, Penner R. TRPM2: a multifunctional ion channel for calcium signalling. J Physiol. 2011;589:1515–1525. doi: 10.1113/jphysiol.2010.201855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Williams A, Sarkar S, Cuddon P, Ttofi EK, Saiki S, Siddiqi FH, Jahreiss L, Fleming A, Pask D, Goldsmith P, O'Kane CJ, Floto RA, Rubinsztein DC. Novel targets for Huntington's disease in an mTOR-independent autophagy pathway. Nature chemical biology. 2008;4:295–305. doi: 10.1038/nchembio.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bialik S, Kimchi A. Lethal weapons: DAP-kinase, autophagy and cell death: DAP-kinase regulates autophagy. Curr Opin Cell Biol. 2010;22:199–205. doi: 10.1016/j.ceb.2009.11.004. [DOI] [PubMed] [Google Scholar]
- 83.Sano R, Reed JC. ER stress-induced cell death mechanisms. Biochim Biophys Acta. 2013;1833:3460–3470. doi: 10.1016/j.bbamcr.2013.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hoyer-Hansen M, Jaattela M. Connecting endoplasmic reticulum stress to autophagy by unfolded protein response and calcium. Cell Death Differ. 2007;14:1576–1582. doi: 10.1038/sj.cdd.4402200. [DOI] [PubMed] [Google Scholar]
- 85.Pattingre S, Tassa A, Qu X, Garuti R, Liang XH, Mizushima N, Packer M, Schneider MD, Levine B. Bcl-2 antiapoptotic proteins inhibit Beclin 1-dependent autophagy. Cell. 2005;122:927–939. doi: 10.1016/j.cell.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 86.Zhang XD, Wang Y, Wu JC, Lin F, Han R, Han F, Fukunaga K, Qin ZH. Down-regulation of Bcl-2 enhances autophagy activation and cell death induced by mitochondrial dysfunction in rat striatum. J Neurosci Res. 2009;87:3600–3610. doi: 10.1002/jnr.22152. [DOI] [PubMed] [Google Scholar]
- 87.Luo S, Rubinsztein DC. Atg5 and Bcl-2 provide novel insights into the interplay between apoptosis and autophagy. Cell Death Differ. 2007;14:1247–1250. doi: 10.1038/sj.cdd.4402149. [DOI] [PubMed] [Google Scholar]
- 88.Tanida I, Sou YS, Ezaki J, Minematsu-Ikeguchi N, Ueno T, Kominami E. HsAtg4B/HsApg4B/autophagin-1 cleaves the carboxyl termini of three human Atg8 homologues and delipidates microtubule-associated protein light chain 3- and GABAA receptor-associated protein-phospholipid conjugates. J Biol Chem. 2004;279:36268–36276. doi: 10.1074/jbc.M401461200. [DOI] [PubMed] [Google Scholar]
- 89.Mizushima N, Yamamoto A, Hatano M, Kobayashi Y, Kabeya Y, Suzuki K, Tokuhisa T, Ohsumi Y, Yoshimori T. Dissection of autophagosome formation using Apg5-deficient mouse embryonic stem cells. J Cell Biol. 2001;152:657–668. doi: 10.1083/jcb.152.4.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Xie Z, Klionsky DJ. Autophagosome formation: core machinery and adaptations. Nat Cell Biol. 2007;9:1102–1109. doi: 10.1038/ncb1007-1102. [DOI] [PubMed] [Google Scholar]
- 91.Xie Z, Nair U, Klionsky DJ. Atg8 controls phagophore expansion during autophagosome formation. Mol Biol Cell. 2008;19:3290–3298. doi: 10.1091/mbc.E07-12-1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Perez-Martin M, Perez-Perez ME, Lemaire SD, Crespo JL. Oxidative stress contributes to autophagy induction in response to endoplasmic reticulum stress in Chlamydomonas reinhardtii. Plant physiology. 2014;166:997–1008. doi: 10.1104/pp.114.243659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Noctor G, Mhamdi A, Chaouch S, Han Y, Neukermans J, Marquez-Garcia B, Queval G, Foyer CH. Glutathione in plants: an integrated overview. Plant Cell Environ. 2012;35:454–484. doi: 10.1111/j.1365-3040.2011.02400.x. [DOI] [PubMed] [Google Scholar]
- 94.Tu BP, Weissman JS. Oxidative protein folding in eukaryotes: mechanisms and consequences. J Cell Biol. 2004;164:341–346. doi: 10.1083/jcb.200311055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Avivar-Valderas A, Salas E, Bobrovnikova-Marjon E, Diehl JA, Nagi C, Debnath J, Aguirre-Ghiso JA. PERK integrates autophagy and oxidative stress responses to promote survival during extracellular matrix detachment. Mol Cell Biol. 2011;31:3616–3629. doi: 10.1128/MCB.05164-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Shen Y, Yang J, Zhao J, Xiao C, Xu C, Xiang Y. The switch from ER stress-induced apoptosis to autophagy via ROS-mediated JNK/p62 signals: A survival mechanism in methotrexate-resistant choriocarcinoma cells. Experimental cell research. 2015;334:207–218. doi: 10.1016/j.yexcr.2015.04.010. [DOI] [PubMed] [Google Scholar]
- 97.Filomeni G, De Zio D, Cecconi F. Oxidative stress and autophagy: the clash between damage and metabolic needs. Cell Death Differ. 2015;22:377–388. doi: 10.1038/cdd.2014.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Levonen AL, Hill BG, Kansanen E, Zhang J, Darley-Usmar VM. Redox regulation of antioxidants, autophagy, and the response to stress: implications for electrophile therapeutics. Free Radic Biol Med. 2014;71:196–207. doi: 10.1016/j.freeradbiomed.2014.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Scherz-Shouval R, Elazar Z. Regulation of autophagy by ROS: physiology and pathology. Trends Biochem Sci. 2011;36:30–38. doi: 10.1016/j.tibs.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 100.Berbey C, Weiss N, Legrand C, Allard B. Transient receptor potential canonical type 1 (TRPC1) operates as a sarcoplasmic reticulum calcium leak channel in skeletal muscle. J Biol Chem. 2009;284:36387–36394. doi: 10.1074/jbc.M109.073221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Selvaraj S, Sun Y, Watt JA, Wang S, Lei S, Birnbaumer L, Singh BB. Neurotoxin-induced ER stress in mouse dopaminergic neurons involves downregulation of TRPC1 and inhibition of AKT/mTOR signaling. The Journal of clinical investigation. 2012;122:1354–1367. doi: 10.1172/JCI61332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Liu M, Liu MC, Magoulas C, Priestley JV, Willmott NJ. Versatile regulation of cytosolic Ca2+ by vanilloid receptor I in rat dorsal root ganglion neurons. J Biol Chem. 2003;278:5462–5472. doi: 10.1074/jbc.M209111200. [DOI] [PubMed] [Google Scholar]
- 103.Turner H, Fleig A, Stokes A, Kinet JP, Penner R. Discrimination of intracellular calcium store subcompartments using TRPV1 (transient receptor potential channel, vanilloid subfamily member 1) release channel activity. Biochem J. 2003;371:341–350. doi: 10.1042/BJ20021381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Gallego-Sandín S, Rodríguez-García A, Alonso MT, García-Sancho J. The endoplasmic reticulum of dorsal root ganglion neurons contains functional TRPV1 channels. J Biol Chem. 2009;284:32591–32601. doi: 10.1074/jbc.M109.019687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Castro J, Aromataris EC, Rychkov GY, Barritt GJ. A small component of the endoplasmic reticulum is required for store-operated Ca2+ channel activation in liver cells: evidence from studies using TRPV1 and taurodeoxycholic acid. Biochem J. 2009;418:553–566. doi: 10.1042/BJ20081052. [DOI] [PubMed] [Google Scholar]
- 106.Olah Z, Szabo T, Karai L, Hough C, Fields RD, Caudle RM, Blumberg PM, Iadarola MJ. Ligand-induced dynamic membrane changes and cell deletion conferred by vanilloid receptor 1. J Biol Chem. 2001;276:11021–11030. doi: 10.1074/jbc.M008392200. [DOI] [PubMed] [Google Scholar]
- 107.Wisnoskey BJ, Sinkins WG, Schilling WP. Activation of vanilloid receptor type I in the endoplasmic reticulum fails to activate store-operated Ca2+ entry. Biochem J. 2003;372:517–528. doi: 10.1042/BJ20021574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kárai LJ, Russell JT, Iadarola MJ, Oláh Z. Vanilloid receptor 1 regulates multiple calcium compartments and contributes to Ca2+-induced Ca2+ release in sensory neurons. J Biol Chem. 2004;279:16377–16387. doi: 10.1074/jbc.M310891200. [DOI] [PubMed] [Google Scholar]
- 109.Mitchell JE, Campbell AP, New NE, Sadofsky LR, Kastelik JA, Mulrennan SA, Compton SJ, Morice AH. Expression and characterization of the intracellular vanilloid receptor (TRPV1) in bronchi from patients with chronic cough. Exp Lung Res. 2005;31:295–306. doi: 10.1080/01902140590918803. [DOI] [PubMed] [Google Scholar]
- 110.Albarran L, Berna-Erro A, Dionisio N, Redondo PC, Lopez E, Lopez JJ, Salido GM, Brull Sabate JM, Rosado JA. TRPC6 participates in the regulation of cytosolic basal calcium concentration in murine resting platelets. Biochim Biophys Acta. 2014;1843:789–796. doi: 10.1016/j.bbamcr.2014.01.014. [DOI] [PubMed] [Google Scholar]
- 111.Ampem PT, Smedlund K, Vazquez G. Pharmacological evidence for a role of the transient receptor potential canonical 3 (TRPC3) channel in endoplasmic reticulum stress-induced apoptosis of human coronary artery endothelial cells. Vascul Pharmacol. 2015 doi: 10.1016/j.vph.2015.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Chen S, He FF, Wang H, Fang Z, Shao N, Tian XJ, Liu JS, Zhu ZH, Wang YM, Wang S, Huang K, Zhang C. Calcium entry via TRPC6 mediates albumin overload-induced endoplasmic reticulum stress and apoptosis in podocytes. Cell Calcium. 2011;50:523–529. doi: 10.1016/j.ceca.2011.08.008. [DOI] [PubMed] [Google Scholar]
- 113.Harding HP, Calfon M, Urano F, Novoa I, Ron D. Transcriptional and translational control in the Mammalian unfolded protein response. Annu Rev Cell Dev Biol. 2002;18:575–599. doi: 10.1146/annurev.cellbio.18.011402.160624. [DOI] [PubMed] [Google Scholar]
- 114.Kim MS, Lee KP, Yang D, Shin DM, Abramowitz J, Kiyonaka S, Birnbaumer L, Mori Y, Muallem S. Genetic and pharmacologic inhibition of the Ca2+ influx channel TRPC3 protects secretory epithelia from Ca2+-dependent toxicity. Gastroenterology. 2011;140:2107–2115. 2115, e2101–2104. doi: 10.1053/j.gastro.2011.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Timmins JM, Ozcan L, Seimon TA, Li G, Malagelada C, Backs J, Backs T, Bassel-Duby R, Olson EN, Anderson ME, Tabas I. Calcium/calmodulin-dependent protein kinase II links ER stress with Fas and mitochondrial apoptosis pathways. The Journal of clinical investigation. 2009;119:2925–2941. doi: 10.1172/JCI38857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Smedlund K, Tano JY, Vazquez G. The constitutive function of native TRPC3 channels modulates vascular cell adhesion molecule-1 expression in coronary endothelial cells through nuclear factor kappaB signaling. Circ Res. 2010;106:1479–1488. doi: 10.1161/CIRCRESAHA.109.213314. [DOI] [PubMed] [Google Scholar]
- 117.Thomas KC, Sabnis AS, Johansen ME, Lanza DL, Moos PJ, Yost GS, Reilly CA. Transient receptor potential vanilloid 1 agonists cause endoplasmic reticulum stress and cell death in human lung cells. The Journal of pharmacology and experimental therapeutics. 2007;321:830–838. doi: 10.1124/jpet.107.119412. [DOI] [PubMed] [Google Scholar]
- 118.Thomas KC, Roberts JK, Deering-Rice CE, Romero EG, Dull RO, Lee J, Yost GS, Reilly CA. Contributions of TRPV1, endovanilloids, and endoplasmic reticulum stress in lung cell death in vitro and lung injury. Am J Physiol Lung Cell Mol Physiol. 2012;302:L111–119. doi: 10.1152/ajplung.00231.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]