Abstract
Along with the increased frequency of implantation, the incidence of cardiac implantable electronic device (CIED) infection, which can have serious or fatal complications, has also increased. Although several successful conservative therapies for CIED infection have been reported, retained infected devices remain a source of relapse, which is closely related to a higher mortality rate. Presently, complete hardware removal is initially recommended for infected CIED patients, and indications for conservative therapy, including continuous administration of antibiotics, require careful consideration.
On the other hand, complete removal is not required for superficial or incisional infection at the device pocket if an infection does not involve the device, but the patient should be closely followed for progression to deeper infection, which would require extraction.
Abbreviations: CIED, cardiac implantable electronic device
Keywords: Cardiac implantable electronic device, Infection, Antibiotic therapy, Device extraction
1. Introduction
Treatment with a cardiac implantable electronic device (CIED), including a permanent pacemaker (PPM) or implantable cardioverter–defibrillator (ICD), is very useful. At present, an ICD is needed not only to rescue survivors of life-threating ventricular arrhythmias, but is also first-line primary prevention to save patients with marked cardiac dysfunction. Moreover, medical technology for resynchronization therapy has advanced, with a resulting increased rate of device implantation [1], [3]. Along with the increase in device implantation, the incidence of CIED infection, which can have serious or fatal complications, has also increased [2], [4], [5], [6], [7]. Prutkin et al. reported that a total of 3390 (1.7%) out of 200,909 implanted ICDs developed a device infection within six months [6]. Other studies have reported a similar rate of CIED infection (0.7–2.2%) [5], [8], [9], [10].
The management of infected CIED patients is difficult, and requires special knowledge and effort by physicians. Intensive treatment of CIED infections is recommended, including administration of appropriate antibiotics and complete removal of the infected device [5], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22]. Consensus reports and statements from the literature also recommend prompt and complete device removal in combination with antimicrobial therapy [22], [23], [24]. In this article, we present the role of conservative therapy for the management of CIED infection.
2. Role of continuous antibiotic therapy
When CIED infection is diagnosed, early complete removal of the infected device is strongly recommended because of the high association with increased mortality. No prospective randomized studies have assessed the value of conservative antibiotic therapy alone, compared with combined complete device removal and administration of appropriate antibiotics. However, some reports cited the poor outcome using conservative antibiotic therapy in patients with CIED infection [11], [25]. About 30 years ago, Lewis et al. reported on treatment of patients with permanent pacemaker infections [26]. Thirty-two patients received conservative antibiotic therapy without removal of the infected pacing system, which included limited debridement and irrigation or aspiration of the infected sites. This conservative therapy failed in all, but one of the 32 patients, and complete removal of the device system was needed in 31. Molina et al. subsequently reported the outcome of management in 38 patients with an infected CIED, including 17 ICDs [27]. Twelve patients received intravenous antibiotics without removal of the CIED, but with relocation to a different area, with or without aspiration of the infected area. Patients who received conservative therapy ultimately all remained infected. Two deaths occurred in these patients. On the other hand, the remaining 26 CIED patients who underwent primary removal of the entire device system were completely cured. No deaths occurred in these patients. These data supported the value of prompt complete device removal in infected CIED patients.
3. Mortality
Although complete removal of an infected CIED is first-line therapy, there can be a risk of device removal complications. It was reported that complications of device extraction were associated with increased mortality in patients with CIED infections at both 30 days (hazard ratio [HR]: 4.33, 95% confidence interval [CI]: 1.47–12.70) and one year (HR: 3.77, 95% CI: 1.88–7.55) [11]. However, delayed device removal was associated with a 3-fold increase in one-year mortality, and antibiotic therapy without device removal was associated with a 7-fold increase in 30-day mortality, compared to immediate device removal in the previous paper. Margey et al. also described outcomes and mortality in infected CIED patients [5]. Of 39 (82%) patients, 32 underwent device extraction, and complete device removal was accomplished in 27 (84%). Of these, none relapsed, and mortality was 7.4% during a median follow-up period of 36 months. Of those managed with only generator removal or conservative therapy, relapse occurred in 8 of 12 (67%), with mortality occurring in 8.4%. Using data on patients with infective endocarditis and a cardiac device, Athan et al reported that 28 of 141 (19.9%) who underwent device removal during the index hospitalization died at one year, compared with 13 of 34 (38.2%) who did not undergo device removal (HR: 0.42, 95% CI: 0.22–0.82), which suggested that device removal was associated with improved survival at one year [12].
4. Possibility of successful conservative therapy
Some patients have severe complicated medical problems and are considered too ill to tolerate aggressive surgical intervention. In addition, some patients refuse surgery, or have a limited life span. In such cases, conservative treatment has been attempted. In 1985, Garcia-Rinaidi et al. successfully treated exposed pacemakers relocated to deeper subfascial planes without relapse of infectious signs in 10 patients [28]. They concluded that subfascial relocation of a properly functioning pacemaker generator should be considered as an alternative to complete replacement of the unit. Hurst et al. also reported that 19 infected or extruded pacemaker patients were treated with local debridement and insertion of a closed irrigation system using solutions of tyloxapol and tobramycin [29]. In order to achieve successful treatment, they insisted on careful debridement of the infected pocket and large pocket space to ensure adequate closure without tension. Using this method, they have been successful in salvaging infected pacemaker pockets, and all 19 patients healed without complications after treatment (the maximum follow-up period was 70 months).
Clinical data on 51 patients with intravascular device-related infections who were administered long-term suppressive antibiotic therapy were also reported [30]. The categories included vascular graft infections in 30 (59%) patients, prosthetic valve endocarditis in 12, pacemaker-related infections in 5, aortic graft infections in 3, central venous catheter infections in 3, and venous filter infection in one. Therapy duration ranged from 3 months to 10 years. Of 41 followed-up patients, 3 (7%) had an infection relapse while on long-term antibiotic therapy. Three patients died, including one who had an infection relapse and died of the infection. Drug side effects occurred in the other 3 patients. The report stated that long-term antibiotic therapy was well-tolerated and efficacious in nonsurgical candidates. Segreti et al. reviewed 18 patients with infected orthopedic prostheses who had been treated with long-term antibiotic therapy. Although side effects related to antibiotics occurred in 22%, 15 (83%) patients with a mean follow-up of 5 years had a good response to suppression of infection and could retain a functional prosthesis [31]. Ortler et al. experienced the case of one epileptic patient implanted with an infected vagus nerve stimulator system who underwent successful open wound treatment without removal of the system [32]. They reported that the deep wound was initially opened and debrided. With continuous antibiotic therapy and daily rinsing with 3% hydrogen peroxide solution and 5% saline, granulation tissue gradually appeared. In this case, relapse of infection never occurred during a follow-up of 1 year. The value of local treatment with 3% hydrogen peroxide for prevention of foreign body infection has been previously reported [33]. Of 990 CIEDs treated with 3% hydrogen peroxide during the implant procedure, 2 (0.2%) developed an infection, which was a significantly reduced infection rate compared to that of CIEDs treated without hydrogen peroxide during implantation (1.83%, p<0.05).
Although conservative therapies might be useful in limited cases, retained infectious devices have a risk of mortality. Additionally, these papers were published more than 10 years ago, and medical technology has advanced since then. The excimer laser has also been used for CIED lead removal in many countries. If possible, initial complete hardware removal is strongly recommended, and the indications for conservative antibiotic therapy for CIED infections should be determined with great caution.
5. Recent studies
In 2010, Satsu et al. reported the use of a vacuum-assisted wound closure (VAC) system as an option for treating an infected device [34]. The VAC system is comprised of polyurethane foam with an evacuation tube, a vacuum pump, and adhesive drape. Treatment with VAC has been reported to be an efficacious modality for chronic and difficult wounds [35], [36]. After opening the wound, the polyurethane is cut and positioned on the defect area, with connection of the evacuation tube. The wound is then covered with an adhesive drape, and suction is continued with a negative pressure of 100 mmHg. The wound is treated by changing the foam every 2 or 3 days until healing occurs, or the incision is sutured when granulation appears. According to this paper, infections never relapsed after treatment with VAC in all 4 patients for 5–15 months, and CIED infection healed without complete removal of the pacemaker system in 2 patients.
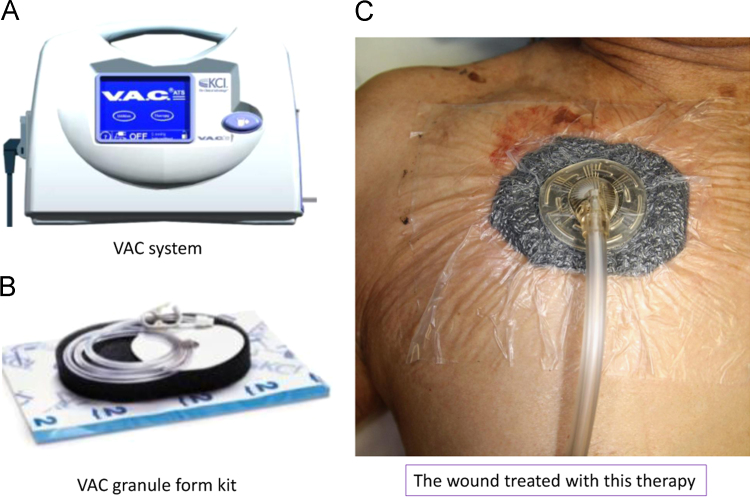
In our hospital, the VAC system is sometimes applied to a large wound after removal of the cardiac device system and debridement of the pocket (Fig. 1).
Fig. 1.
VAC system (Kinetic Concepts, Inc., San Antonio, TX, USA). The VAC system comprises of a sponge with an evacuation tube, an adhesive drape, and a vacuum pump (panels A and B). The sponge is cut and positioned on the defect area with connection of the evacuation tube. The wound is covered with the adhesive drape, and suction is continued with negative pressure (panel C).
6. Superficial or incisional infection
According to the Heart Rhythm Society expert consensus report, CIED removal is not recommended for a superficial or incisional infection without involvement of the device or lead (class III indication) (Fig. 2) [24]. However, it is difficult to distinguish whether a superficial pocket infection might have extended through the device. It was reported that the intravascular parts of leads yielded positive cultures in 79% of 105 study patients who had local pocket complications, which were associated with a risk of progression to systemic infection [37]. Chamis et al. reported no local signs or symptoms related to pocket infection in 60% of patients (9 of 15) with confirmed CIED infections [38]. When superficial or incisional infection of a CIED occurs, conservative antibiotic therapy may be administered as needed, and the patient should be closely followed for progression to a deeper infection, which would require extraction.
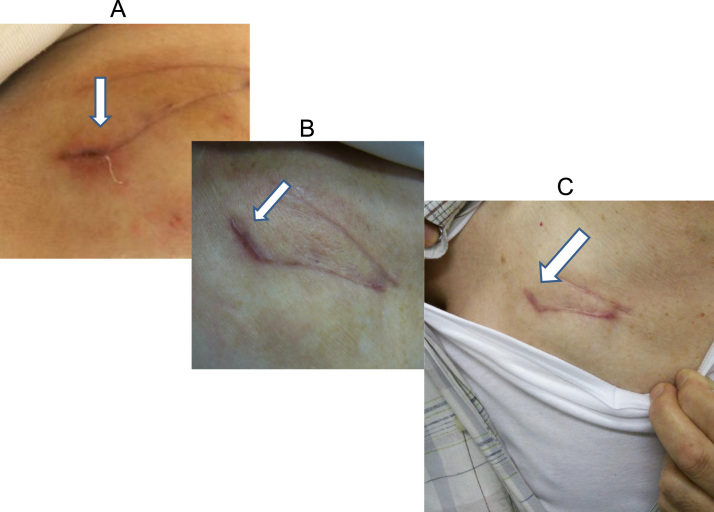
Fig. 2.
A case of superficial incisional infection. Fourteen days after an operation to exchange a pacemaker device, wound dehiscence was observed at the sutured incision line (panel A). Inflammatory markers became elevated, and intravenous antibiotics were administered for 11 days in conjunction with daily wound irrigation. About one month later, the incisional wound was closed, with complete resolution of inflammatory markers (panel B). There was no fever or inflammatory marker elevation during a follow-up period of 6 months. The incisional wound remained clear (panel C).
Several recent studies reported that fluorodeoxyglucose positron emission tomography–computed tomography (FDG-PET/CT) could diagnose CIED infections [13], [39]. In some suspected CIED cases, this new imaging modality may be useful in differentiating a superficial from a systemic infection, and may lead to appropriate therapy.
7. Conclusion
Although several successful conservative therapies for CIED infection have been reported, retained infectious devices remain a source of relapse, which is closely related to a higher mortality rate. Presently, complete hardware removal is initially recommended for infected CIED patients, and the indication of conservative therapy for CIED infections should be determined with great caution.
The absolute rate of device infection has recently increased. Further strategies for reducing CIED infection should be considered.
Conflict of interests
All authors declare no conflict of interest related to this study.
References
- 1.Goldberger Z., Lampert R. Implantable cardioverter–defibrillators: Expanding indications and technologies. J Am Med Assoc. 2006;295:809–818. doi: 10.1001/jama.295.7.809. [DOI] [PubMed] [Google Scholar]
- 2.Uslan D.Z., Baddour L.M. Cardiac device infections: getting to the heart of the matter. Curr Opin Infect Dis. 2006;19:345–348. doi: 10.1097/01.qco.0000235160.78302.24. [DOI] [PubMed] [Google Scholar]
- 3.Uslan D.Z., Sohail M.R., St. Sauver J.L. Permanent pacemaker and implantable cardioverter defibrillator infection: a population-based study. Arch Intern Med. 2007;167:669–675. doi: 10.1001/archinte.167.7.669. [DOI] [PubMed] [Google Scholar]
- 4.Voigt A., Shalaby A., Saba S. Rising rates of cardiac rhythm management device infections in the United States: 1996 through 2003. J Am Coll Cardiol. 2006;48:590–591. doi: 10.1016/j.jacc.2006.05.016. [DOI] [PubMed] [Google Scholar]
- 5.Margey R., McCann H., Blake G. Contemporary management of and outcomes from cardiac device related infections. Europace. 2010;12:64–70. doi: 10.1093/europace/eup362. [DOI] [PubMed] [Google Scholar]
- 6.Prutkin J.M., Reynolds M.R., Bao H. Rates of and factors associated with infection in 200 909 medicare implantable cardioverter–defibrillator implants: results from the national cardiovascular data registry. Circulation. 2014;130:1037–1043. doi: 10.1161/CIRCULATIONAHA.114.009081. [DOI] [PubMed] [Google Scholar]
- 7.Alter P., Waldhans S., Plachta E. Complications of implantable cardioverter defibrillator therapy in 440 consecutive patients. Pacing Clin Electrophysiol. 2005;28:926–932. doi: 10.1111/j.1540-8159.2005.00195.x. [DOI] [PubMed] [Google Scholar]
- 8.Nery P.B., Fernandes R., Nair G.M. Device-related infection among patients with pacemakers and implantable defibrillators: incidence, risk factors, and consequences. J Cardiovasc Electrophysiol. 2010;21:786–790. doi: 10.1111/j.1540-8167.2009.01690.x. [DOI] [PubMed] [Google Scholar]
- 9.Klug D., Balde M., Pavin D. Risk factors related to infections of implanted pacemakers and cardioverter–defibrillators: results of a large prospective study. Circulation. 2007;116:1349–1355. doi: 10.1161/CIRCULATIONAHA.106.678664. [DOI] [PubMed] [Google Scholar]
- 10.Lekkerkerker J.C., van Nieuwkoop C., Trines S.A. Risk factors and time delay associated with cardiac device infections: leiden device registry. Heart. 2009;95:715–720. doi: 10.1136/hrt.2008.151985. [DOI] [PubMed] [Google Scholar]
- 11.Le K.Y., Sohail M.R., Friedman P.A. Impact of timing of device removal on mortality in patients with cardiovascular implantable electronic device infections. Heart Rhythm. 2011;8:1678–1685. doi: 10.1016/j.hrthm.2011.05.015. [DOI] [PubMed] [Google Scholar]
- 12.Athan E., Chu V.H., Tattevin P. Clinical characteristics and outcome of infective endocarditis involving implantable cardiac devices. J Am Med Assoc. 2012;307:1727–1735. doi: 10.1001/jama.2012.497. [DOI] [PubMed] [Google Scholar]
- 13.Sarrazin J.F., Philippon F., Tessier M. Usefulness of fluorine-18 positron emission tomography/computed tomography for identification of cardiovascular implantable electronic device infections. J Am Coll Cardiol. 2012;59:1616–1625. doi: 10.1016/j.jacc.2011.11.059. [DOI] [PubMed] [Google Scholar]
- 14.Durante-Mangoni E., Mattucci I., Agrusta F. Current trends in the management of cardiac implantable electronic device (CIED) infections. Intern Emerg Med. 2013;8:465–476. doi: 10.1007/s11739-012-0797-6. [DOI] [PubMed] [Google Scholar]
- 15.Habib A., Le K.Y., Baddour L.M. Predictors of mortality in patients with cardiovascular implantable electronic device infections. Am J Cardiol. 2013;111:874–879. doi: 10.1016/j.amjcard.2012.11.052. [DOI] [PubMed] [Google Scholar]
- 16.Nielsen J.C., Gerdes J.C., Varma N. Infected cardiac-implantable electronic devices: prevention, diagnosis, and treatment. Eur Heart J. 2015;36:2484–2490. doi: 10.1093/eurheartj/ehv060. [DOI] [PubMed] [Google Scholar]
- 17.Hauser R.G., Katsiyiannis W.T., Gornick C.C. Deaths and cardiovascular injuries due to device-assisted implantable cardioverter–defibrillator and pacemaker lead extraction. Europace. 2010;12:395–401. doi: 10.1093/europace/eup375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sohail M.R., Uslan D.Z., Khan A.H. Infective endocarditis complicating permanent pacemaker and implantable cardioverter–defibrillator infection. Mayo Clin Proc. 2008;83:46–53. doi: 10.4065/83.1.46. [DOI] [PubMed] [Google Scholar]
- 19.Tarakji K.G., Chan E.J., Cantillon D.J. Cardiac implantable electronic device infections: presentation, management, and patient outcomes. Heart Rhythm. 2010;7:1043–1047. doi: 10.1016/j.hrthm.2010.05.016. [DOI] [PubMed] [Google Scholar]
- 20.Knigina L., Kuhn C., Kutschka I. Treatment of patients with recurrent or persistent infection of cardiac implantable electronic devices. Europace. 2010;12:1275–1281. doi: 10.1093/europace/euq192. [DOI] [PubMed] [Google Scholar]
- 21.Parry G., Goudevenos J., Jameson S. Complications associated with retained pacemaker leads. Pacing Clin Electrophysiol. 1991;14:1251–1257. doi: 10.1111/j.1540-8159.1991.tb02864.x. [DOI] [PubMed] [Google Scholar]
- 22.Baddour L.M., Epstein A.E., Erickson C.C. Update on cardiovascular implantable electronic device infections and their management: a scientific statement from the American Heart Association. Circulation. 2010;121:458–477. doi: 10.1161/CIRCULATIONAHA.109.192665. [DOI] [PubMed] [Google Scholar]
- 23.Baddour L.M., Bettmann M.A., Bolger A.F. Nonvalvular cardiovascular device-related infections. Circulation. 2003;108:2015–2031. doi: 10.1161/01.CIR.0000093201.57771.47. [DOI] [PubMed] [Google Scholar]
- 24.Wilkoff B.L., Love C.J., Byrd C.L. Transvenous lead extraction: Heart Rhythm Society expert consensus on facilities, training, indications, and patient management: this document was endorsed by the American Heart Association (AHA) Heart Rhythm. 2009;6:1085–1104. doi: 10.1016/j.hrthm.2009.05.020. [DOI] [PubMed] [Google Scholar]
- 25.Bracke F.A., Meijer A., van Gelder L.M. Pacemaker lead complications: When is extraction appropriate and what can we learn from published data? Heart. 2001;85:254–259. doi: 10.1136/heart.85.3.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lewis A.B., Hayes D.L., Holmes D.R., Jr. Update on infections involving permanent pacemakers. Characterization and management. J Thorac Cardiovasc Surg. 1985;89:758–763. [PubMed] [Google Scholar]
- 27.Molina J.E. Undertreatment and overtreatment of patients with infected antiarrhythmic implantable devices. Ann Thorac Surg. 1997;63:504–509. doi: 10.1016/s0003-4975(96)01033-8. [DOI] [PubMed] [Google Scholar]
- 28.Garcia-Rinaldi R., Revuelta J.M., Bonnington L. The exposed cardiac pacemaker. Treatment by subfascial pocket relocation. J Thorac Cardiovasc Surg. 1985;89:136–141. [PubMed] [Google Scholar]
- 29.Hurst L.N., Evans H.B., Windle B. The salvage of infected cardiac pacemaker pockets using a closed irrigation system. Pacing Clin Electrophysiol. 1986;9:785–792. doi: 10.1111/j.1540-8159.1986.tb06628.x. [DOI] [PubMed] [Google Scholar]
- 30.Baddour L.M. Long-term suppressive antimicrobial therapy for intravascular device-related infections. Am J Med Sci. 2001;322:209–212. doi: 10.1097/00000441-200110000-00011. [DOI] [PubMed] [Google Scholar]
- 31.Segreti J., Nelson J.A., Trenholme G.M. Prolonged suppressive antibiotic therapy for infected orthopedic prostheses. Clin Infect Dis. 1998;27:711–713. doi: 10.1086/514951. [DOI] [PubMed] [Google Scholar]
- 32.Ortler M., Luef G., Kofler A. Deep wound infection after vagus nerve stimulator implantation: treatment without removal of the device. Epilepsia. 2001;42:133–135. doi: 10.1046/j.1528-1157.2001.23800.x. [DOI] [PubMed] [Google Scholar]
- 33.Alt E., Leipold F., Milatovic D. Hydrogen peroxide for prevention of bacterial growth on polymer biomaterials. Ann Thorac Surg. 1999;68:2123–2128. doi: 10.1016/s0003-4975(99)00832-2. [DOI] [PubMed] [Google Scholar]
- 34.Satsu T., Onoe M. Vacuum-assisted wound closure for pacemaker infection. Pacing Clin Electrophysiol. 2010;33:426–430. doi: 10.1111/j.1540-8159.2009.02661.x. [DOI] [PubMed] [Google Scholar]
- 35.Argenta L.C., Morykwas M.J. Vacuum-assisted closure: a new method for wound control and treatment: clinical experience. Ann Plast Surg. 1997;38:563–576. (discussion 577) [PubMed] [Google Scholar]
- 36.Klug D., Lacroix D., Savoye C. Systemic infection related to endocarditis on pacemaker leads: clinical presentation and management. Circulation. 1997;95:2098–2107. doi: 10.1161/01.cir.95.8.2098. [DOI] [PubMed] [Google Scholar]
- 37.Klug D., Wallet F., Lacroix D. Local symptoms at the site of pacemaker implantation indicate latent systemic infection. Heart. 2004;90:882–886. doi: 10.1136/hrt.2003.010595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chamis A.L., Peterson G.E., Cabell C.H. Staphylococcus aureus bacteremia in patients with permanent pacemakers or implantable cardioverter–defibrillators. Circulation. 2001;104:1029–1033. doi: 10.1161/hc3401.095097. [DOI] [PubMed] [Google Scholar]
- 39.Ploux S., Riviere A., Amraoui S. Positron emission tomography in patients with suspected pacing system infections may play a critical role in difficult cases. Heart Rhythm. 2011;8:1478–1481. doi: 10.1016/j.hrthm.2011.03.062. [DOI] [PubMed] [Google Scholar]