Fig 2. KTAMK-mutant RUNX1 retains its ability to rescue the in vitro haematopoietic defects observed in Runx1-defficient mouse ES cells.
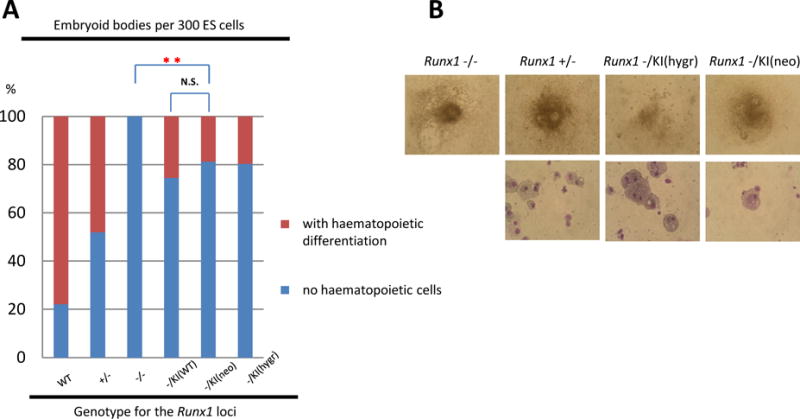
(A) Results of embryoid body (EB) differentiation. Incidence of haematopoietic differentiation of day-14 EBs derived from embryonic stem (ES) cell clones was determined in a representative experiment using the following genotypes: wild-type, heterozygous for Runx1-disruption (+/−), homozygous for the disruption (−/−), one knock-in allele for wild-type Runx1 cDNA (−/KI(WT)), one knock-in allele for KTAMK-mutant at the hygromycin allele (−/KI(hygr)), and one knock-in allele for KTAMK-mutant at the neomycin allele (−/KI(neo)). Red sections of columns indicate the proportion of grown embryoid bodies (EBs) with haematopoietic differentiation and blue sections represent the proportion of those without haematopoietic elements. ES clones of KTAMK-mutant knock-in allele developed into haematopoietic cells in vitro as did control clones. Statistically significant differences are shown as P values (**P<0.001 t test). N.S., not statistically different. (B) Appearance of representative day-14 EBs derived from each of the ES cell clones (top row). Morphology of the haematopoietic cells developed from ES cells of KTAMK-mutant knock-in clones showed no remarkable abnormalities of component cells examined with May-Grünwald-Giemsa staining (bottom row). Original magnifications: top row, ×20; bottom row, ×132.
