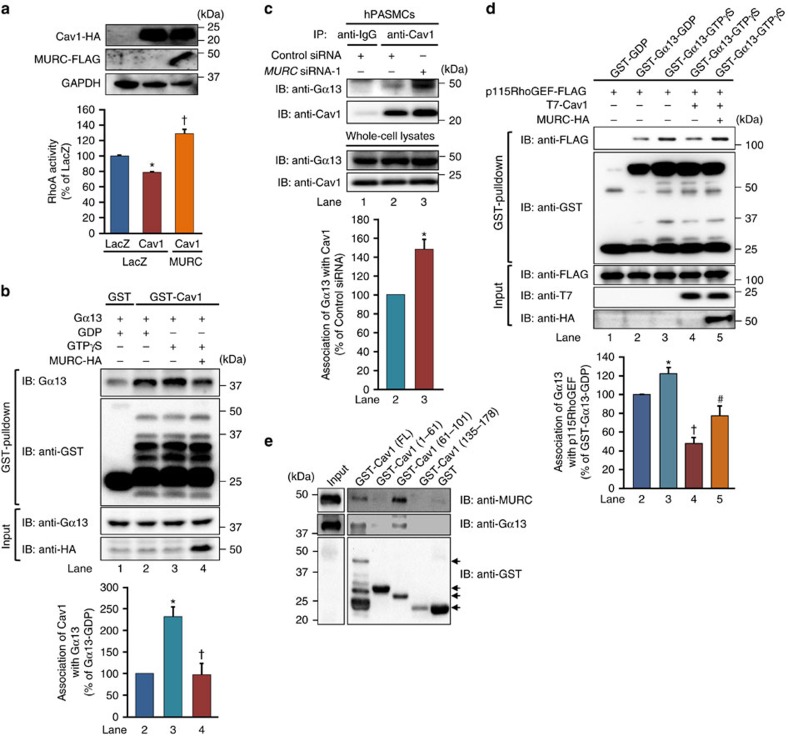
Figure 6. Inhibition of the association of Cav1 with Gα13 by MURC.
(a) RhoA activity was assessed in hPASMCs transduced with LacZ, Cav1-HA and Cav1-HA and MURC-FLAG (n=4 per group). hPASMCs were infected with a hygromycin-resistant retrovirus expressing LacZ and Cav1-HA. After selection using hygromycin, hPASMCs were infected with a puromycin-resistant retrovirus expressing LacZ and MURC-FLAG, and subsequently selected using puromycin. *P<0.05 compared with LacZ+LacZ, †P<0.05 compared with LacZ+Cav1. (b) COS cells were transfected with pcDNA3.1-hMURC-HA and/or pcDNA3.1-hGα13. The COS cell lysates incubated with GDP or GTPγS and GST pulldown was performed with GST-fusion Cav1 conjugated to glutathione-Sepharose beads. Precipitated proteins were blotted with anti-Gα13 and anti-GST antibodies (n=3 per group). *P<0.05 compared with Gα13-GDP+Cav1, †P<0.05 compared with Gα13-GTPγS+Cav1. (c) The association of Gα13 with Cav1 was assessed in hPASMCs transfected with control siRNA or MURC siRNA (n=3 per group). *P<0.05 compared with control siRNA. (d) GST-fusion Gα13 conjugated to glutathione-Sepharose beads was preloaded with GDP or GTPγS. GST pulldown was performed with COS cell lysates transfected with plasmids expressing the indicated proteins. Precipitated proteins were blotted with anti-FLAG and anti-GST antibodies (n=5 per group). *P<0.05 compared with Gα13-GDP+p115RhoGEF, †P<0.05 compared with Gα13-GTPγS+p115RhoGEF, #P<0.05 compared with Gα13-GTPγS+p115RhoGEF+Cav1. (e) Each domain of Cav1 (FL: full length, 1–61: C-terminal domain, 61–101: oligomerization domain, 135–178: N-terminal domain) tagged with GST was conjugated to glutathione-Sepharose beads, and incubated with lysates from COS cells transfected with pcDNA3.1-hMURC-FLAG or pcDNA3.1-hGα13. Pulled-down protein was blotted with anti-MURC or anti-Gα13 antibodies. Arrows indicate each GST-fusion protein. Data are presented as mean±s.e.m. Uncropped images of blots are shown in Supplementary Fig. 6.