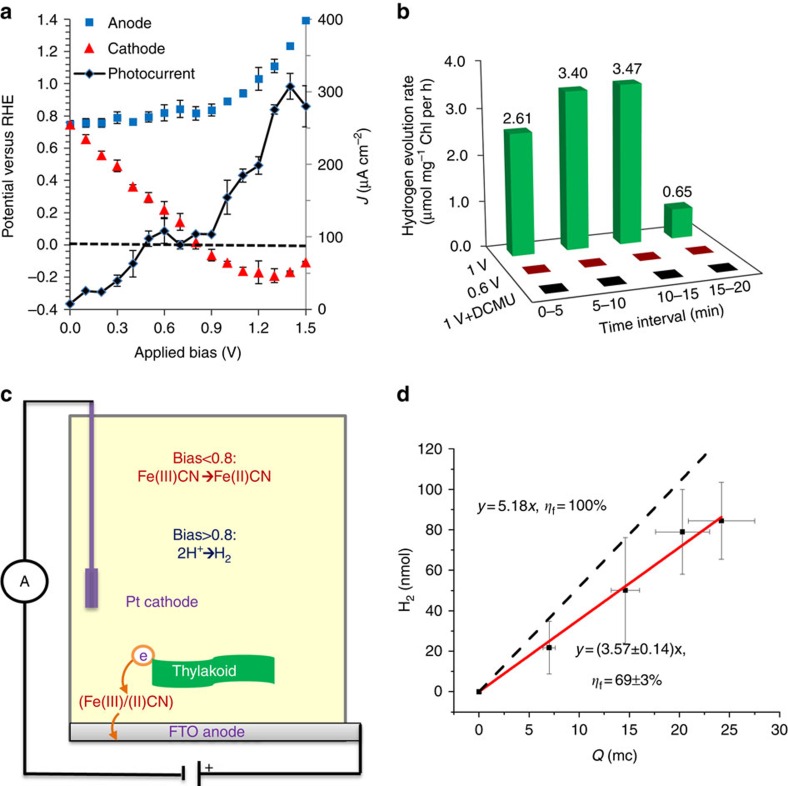
Figure 6. Two-electrode mode measurements.
(a) Photocurrent (black curve) and anode (blue squares) and cathode (red triangles) potentials as a function of the applied bias between the anode and cathode. The dashed line represents a potential of 0 VRHE. The photocurrent was measured under exposure to solar-simulated light in a cell containing buffer A solution with a thylakoid content of 0.1 mg Chl and Fe(III)CN concentration of 3 mM. The error bars represent the s.d. over three independent measurements (b) Hydrogen evolution rate at the cathode under a bias of 0.6 V (red bars), 1.0 V (green bars) and 1.0 V with the addition of 0.5 mM DCMU. (c) Schematic illustration of the proposed electron transfer pathway in the BPEC cell. When the applied bias is lower than 0.8 V the cathode reaction is dominated by cyclic electron transfer of the Fe(III)CN/Fe(II)CN couple, whereas above 0.8 V proton reduction to hydrogen prevails. (d) H2 production as a function of the charge that was transferred between the anode and cathode. Dashed black line corresponds to a Faradic efficiency of 100%. The error bars represent the s.d. over four independent measurements.