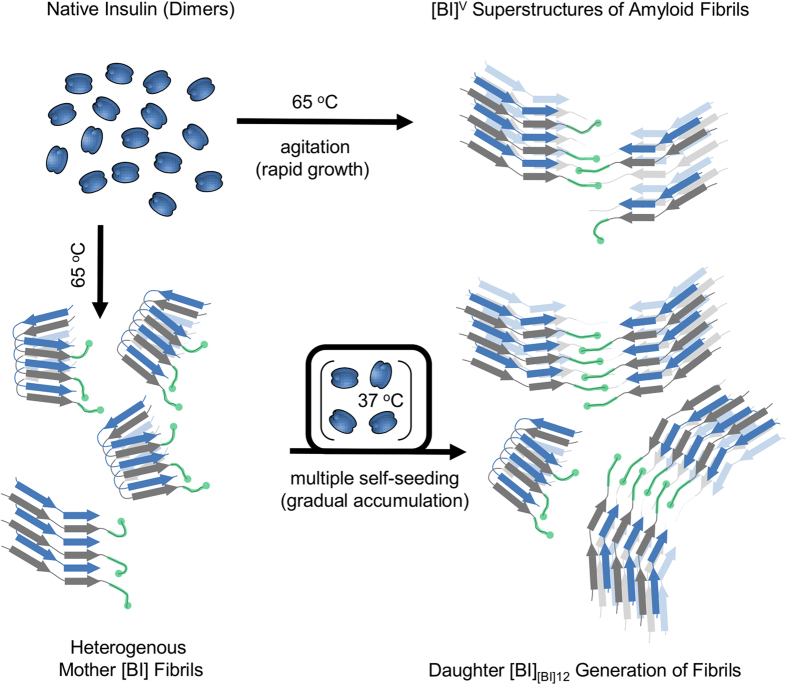
Figure 7. Two different pathways of the self-assembly of chiral superstructures of insulin amyloid fibrils.
Chiral superstructures of insulin amyloid fibrils ([BI]V – marked as “open” stacks of beta-sheets composed of alternate blue and grey strands corresponding to A and B chains of insulin) form rapidly upon intensive agitation of heated solutions of dimeric bovine insulin. Tiny amounts of [BI]V-like structures are likely to form also during insulin fibrillation under quiescent conditions when regular singly dispersed [BI] fibrils (marked as “folded” stacks of beta-sheets) are predominantly formed. Since different elongation rates of [BI]V and [BI] favor the former phenotype upon multiple rounds of self-seeding under quiescent conditions, chiral superstructures of insulin amyloid (presumably bound together by B-chain C-termini – marked in green color) dominate in the following generations of daughter fibrils. The rapid growth of agitation-induced fibrils is likely to result in high concentrations of structural defects rendering thus formed superstructures less rigid and more vulnerable to sonication compared to the chiral variants slowly accumulating upon repeated seeding.