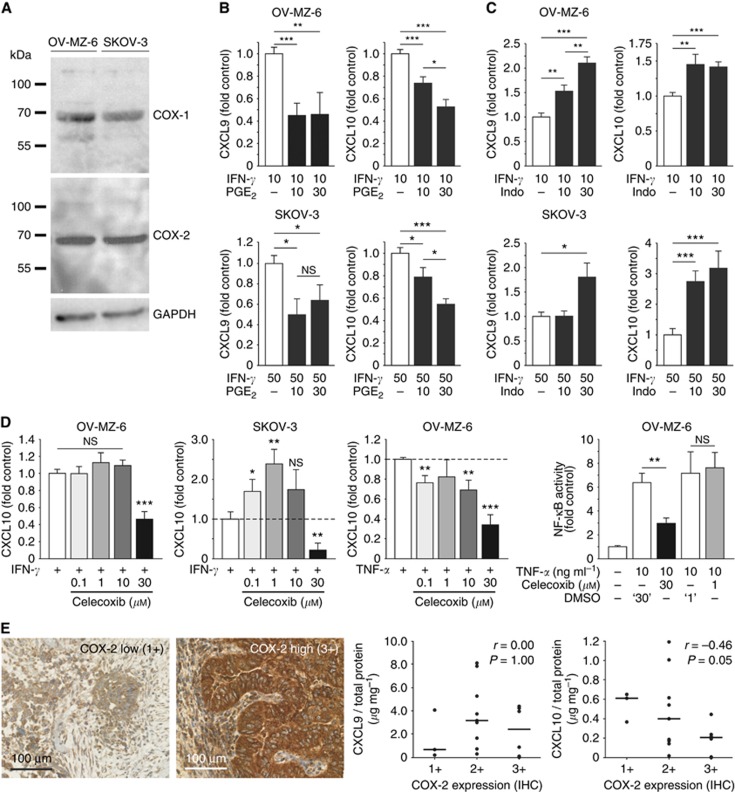
Figure 5.
Modulation of CXCL9 and CXCL10 release from human ovarian cells by the COX system. (A) Western blots showing intrinsic COX-1 and COX-2 expression in OV-MZ-6 and SKOV-3 cells. (B) OV-MZ-6 and SKOV-3 cells were stimulated with increasing concentrations of PGE2 (10 and 30 μM) for 30 min before adding IFN-γ. In both cell lines, PGE2 suppressed IFN-γ-induced CXCL9 and CXCL10 release up to ∼50%. (C) The unselective COX inhibitor indomethacin was added 30 min before IFN-γ on OV-MZ-6 or SKOV-3 cells and significantly enhanced the secretion of both chemokines. (D) Differential effect of the COX-2-specific inhibitor celecoxib on CXCL10 secretion from human ovarian cancer cells. In OV-MZ-6 cells, there was no increase in CXCL10 secretion, in contrast to the effect seen for COX inhibition by indomethacin. In SKOV-3 cells, there was an increase in CXCL10 release only at low celecoxib concentrations (0.1 and 1 μM). In both cell lines, there was a significant inhibition of chemokine release at high (30 μM) celecoxib concentrations. NF-κB activity measured by a reporter gene assay is increased approximately sevenfold 4 h after stimulation with 10 ng ml−1 TNF-α in OV-MZ-6 cells. Celecoxib (1 μM) did not have any effect on NF-κB activity compared with vehicle control (‘1'), whereas 30 μM celecoxib significantly reduces TNF-α-induced NF-κB activity. (E) Correlation of COX-2 expression and CXCL9 and CXCL10 expression in human ovarian cancer. COX-2 expression was assessed semi-quantitatively by immunohistochemistry in primary high-grade serous ovarian cancer samples. Correlation of COX-2 expression with the intratumoral chemokine concentration measured by ELISA (n=18) reveals no correlation between COX-2 and CXCL9, but a significant inverse correlation with CXCL10. Scale bars in E indicate median values. Asterisks mark significant differences compared with controls unless indicated otherwise.