Figure 5.
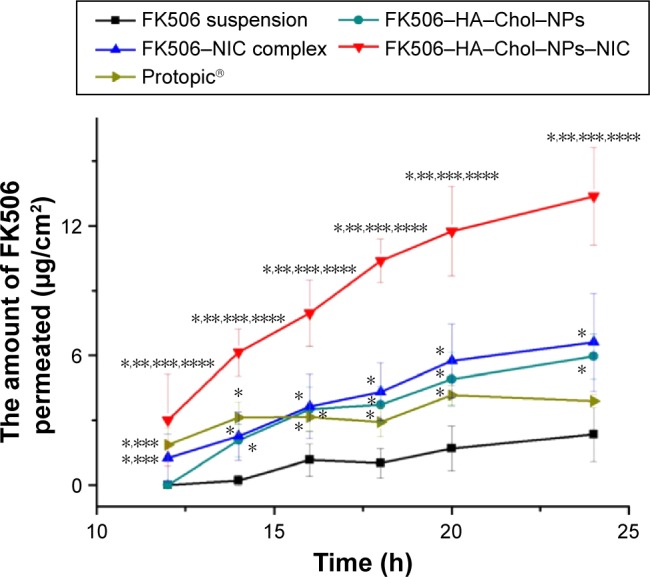
In vitro skin percutaneous studies of FK506 from different vehicles.
Notes: Data represent mean ± SD, n=4 per group. *Significantly different (P<0.05) in comparison with FK506 aqueous suspension. **Significantly different (P<0.05) in comparison with Protopic, 0.1%, w/w. ***Significantly different (P<0.05) in comparison with FK506–NPs. ****Significantly different (P<0.05) in comparison with FK506–NIC solution. The concentration of FK506 in each formulation was 1 mg/mL.
Abbreviations: FK506, tacrolimus; FK506–HA–Chol–NPs, tacrolimus-loaded hyaluronic acid–cholesterol nanoparticles; FK506–HA–Chol–NPs–NIC, tacrolimus-loaded hyaluronic acid–cholesterol nanoparticles containing nicotinamide; FK506–NIC complex, tacrolimus–nicotinamide complex; FK506–NPs, tacrolimus–nanoparticles complex; h, hours; SD, standard deviation.
