Abstract
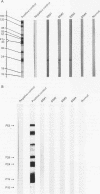
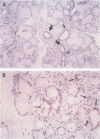
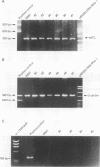
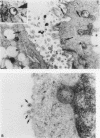
AIMS: To investigate the possibility of an immune response to retroviral antigens or of detecting retrovirus in Sjögren's syndrome. METHODS: Retroviruses were sought in labial salivary glands and peripheral blood mononuclear cells from patients with Sjögren's syndrome by immunoblotting assay, immunohistochemical assay, polymerase chain reaction (PCR), reverse transcriptase (RT) activity assay, and transmission electron microscopy. RESULTS: Sera from five of 15 patients with Sjögren's syndrome (33%) reacted against p24 group specific antigen (gag) of human immunodeficiency virus (HIV). Labial salivary gland biopsy specimens from seven of the 15 patients with Sjögren's syndrome (47%) contained an epithelial cytoplasmic protein reactive with a monoclonal antibody to p24 of HIV. PCR was performed to detect HIV and human T lymphotropic virus type I (HTLV-I) genes from salivary gland tissues and peripheral blood mononuclear cells from patients with Sjögren's syndrome. Mn2+ dependent, Mg2+ independent RT activity was detected in the salivary gland tissues in three of 10 patients. A-type-like retroviral particles were observed in epithelial cells of salivary glands by transmission electron microscopy. Target genes for HIV and HTLV-I were not found in any of the salivary gland tissues or peripheral blood mononuclear cells from Sjögren's syndrome patients. CONCLUSIONS: The data suggest the presence of an unknown retrovirus similar to HIV in the salivary gland which might be involved in the pathogenesis of a subpopulation in Sjögren's syndrome.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adamson T. C., 3rd, Fox R. I., Frisman D. M., Howell F. V. Immunohistologic analysis of lymphoid infiltrates in primary Sjogren's syndrome using monoclonal antibodies. J Immunol. 1983 Jan;130(1):203–208. [PubMed] [Google Scholar]
- Alizon M., Wain-Hobson S., Montagnier L., Sonigo P. Genetic variability of the AIDS virus: nucleotide sequence analysis of two isolates from African patients. Cell. 1986 Jul 4;46(1):63–74. doi: 10.1016/0092-8674(86)90860-3. [DOI] [PubMed] [Google Scholar]
- Banki K., Maceda J., Hurley E., Ablonczy E., Mattson D. H., Szegedy L., Hung C., Perl A. Human T-cell lymphotropic virus (HTLV)-related endogenous sequence, HRES-1, encodes a 28-kDa protein: a possible autoantigen for HTLV-I gag-reactive autoantibodies. Proc Natl Acad Sci U S A. 1992 Mar 1;89(5):1939–1943. doi: 10.1073/pnas.89.5.1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brookes S. M., Pandolfino Y. A., Mitchell T. J., Venables P. J., Shattles W. G., Clark D. A., Entwistle A., Maini R. N. The immune response to and expression of cross-reactive retroviral gag sequences in autoimmune disease. Br J Rheumatol. 1992 Nov;31(11):735–742. doi: 10.1093/rheumatology/31.11.735. [DOI] [PubMed] [Google Scholar]
- Burke D. S., Brundage J. F., Redfield R. R., Damato J. J., Schable C. A., Putman P., Visintine R., Kim H. I. Measurement of the false positive rate in a screening program for human immunodeficiency virus infections. N Engl J Med. 1988 Oct 13;319(15):961–964. doi: 10.1056/NEJM198810133191501. [DOI] [PubMed] [Google Scholar]
- Davies J. W., Cocking E. C. Liquid scintillation counting of 14C and 3H samples using glass-fibre or filter-paper discs. Biochim Biophys Acta. 1966 Feb 28;115(2):511–513. doi: 10.1016/0304-4165(66)90456-9. [DOI] [PubMed] [Google Scholar]
- Díaz-Jouanen E., Ruíz-Argüelles G. J., Vega-Ortíz J. M., Villareal G., Alarcón-Segovia D. From benign polyclonal to malignant monoclonal lymphoproliferation in a patient with primary Sjögren's syndrome. Arthritis Rheum. 1981 Jun;24(6):850–853. doi: 10.1002/art.1780240613. [DOI] [PubMed] [Google Scholar]
- Eguchi K., Matsuoka N., Ida H., Nakashima M., Sakai M., Sakito S., Kawakami A., Terada K., Shimada H., Kawabe Y. Primary Sjögren's syndrome with antibodies to HTLV-I: clinical and laboratory features. Ann Rheum Dis. 1992 Jun;51(6):769–776. doi: 10.1136/ard.51.6.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fauci A. S. The human immunodeficiency virus: infectivity and mechanisms of pathogenesis. Science. 1988 Feb 5;239(4840):617–622. doi: 10.1126/science.3277274. [DOI] [PubMed] [Google Scholar]
- Flescher E., Talal N. Do viruses contribute to the development of Sjögren's syndrome? Am J Med. 1991 Mar;90(3):283–285. [PubMed] [Google Scholar]
- Fox R. I., Howell F. V., Bone R. C., Michelson P. Primary Sjogren syndrome: clinical and immunopathologic features. Semin Arthritis Rheum. 1984 Nov;14(2):77–105. doi: 10.1016/0049-0172(84)90001-5. [DOI] [PubMed] [Google Scholar]
- Fox R. I., Robinson C. A., Curd J. G., Kozin F., Howell F. V. Sjögren's syndrome. Proposed criteria for classification. Arthritis Rheum. 1986 May;29(5):577–585. doi: 10.1002/art.1780290501. [DOI] [PubMed] [Google Scholar]
- Freimark B., Lanier L., Phillips J., Quertermous T., Fox R. Comparison of T cell receptor gene rearrangements in patients with large granular T cell leukemia and Felty's syndrome. J Immunol. 1987 Mar 15;138(6):1724–1729. [PubMed] [Google Scholar]
- Gallo R. C., Mann D., Broder S., Ruscetti F. W., Maeda M., Kalyanaraman V. S., Robert-Guroff M., Reitz M. S., Jr Human T-cell leukemia-lymphoma virus (HTLV) is in T but not B lymphocytes from a patient with cutaneous T-cell lymphoma. Proc Natl Acad Sci U S A. 1982 Sep;79(18):5680–5683. doi: 10.1073/pnas.79.18.5680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garry R. F., Fermin C. D., Hart D. J., Alexander S. S., Donehower L. A., Luo-Zhang H. Detection of a human intracisternal A-type retroviral particle antigenically related to HIV. Science. 1990 Nov 23;250(4984):1127–1129. doi: 10.1126/science.1701273. [DOI] [PubMed] [Google Scholar]
- Horai S., Hayasaka K. Intraspecific nucleotide sequence differences in the major noncoding region of human mitochondrial DNA. Am J Hum Genet. 1990 Apr;46(4):828–842. [PMC free article] [PubMed] [Google Scholar]
- Inagaki Y., Jinno Y., Hamasaki Y., Ueki H. Higher incidences of anti-SS-A/Ro and anti-SS-B/La autoantibodies in Japanese patients with autoimmune disorders--studies of antigens and antibodies using the immunoblotting method. Arch Dermatol Res. 1989;281(2):89–94. doi: 10.1007/BF00426584. [DOI] [PubMed] [Google Scholar]
- Josephson S. L., Swack N. S., Ramirez M. T., Hausler W. J., Jr Investigation of atypical western blot (immunoblot) reactivity involving core proteins of human immunodeficiency virus type. J Clin Microbiol. 1989 May;27(5):932–937. doi: 10.1128/jcm.27.5.932-937.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopelman R. G., Zolla-Pazner S. Association of human immunodeficiency virus infection and autoimmune phenomena. Am J Med. 1988 Jan;84(1):82–88. doi: 10.1016/0002-9343(88)90012-5. [DOI] [PubMed] [Google Scholar]
- Kowalski M., Ardman B., Basiripour L., Lu Y. C., Blohm D., Haseltine W., Sodroski J. Antibodies to CD4 in individuals infected with human immunodeficiency virus type 1. Proc Natl Acad Sci U S A. 1989 May;86(9):3346–3350. doi: 10.1073/pnas.86.9.3346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyanagi Y., Miles S., Mitsuyasu R. T., Merrill J. E., Vinters H. V., Chen I. S. Dual infection of the central nervous system by AIDS viruses with distinct cellular tropisms. Science. 1987 May 15;236(4803):819–822. doi: 10.1126/science.3646751. [DOI] [PubMed] [Google Scholar]
- Krieg A. M., Steinberg A. D. Retroviruses and autoimmunity. J Autoimmun. 1990 Apr;3(2):137–166. doi: 10.1016/0896-8411(90)90137-h. [DOI] [PubMed] [Google Scholar]
- Kuff E. L., Lueders K. K. The intracisternal A-particle gene family: structure and functional aspects. Adv Cancer Res. 1988;51:183–276. doi: 10.1016/s0065-230x(08)60223-7. [DOI] [PubMed] [Google Scholar]
- Lelie P. N., van der Poel C. L., Reesink H. W. Interpretation of isolated HIV anti-p24 reactivity in Western blot analysis. Lancet. 1987 Mar 14;1(8533):632–632. doi: 10.1016/s0140-6736(87)90274-1. [DOI] [PubMed] [Google Scholar]
- Löwer R., Boller K., Hasenmaier B., Korbmacher C., Müller-Lantzsch N., Löwer J., Kurth R. Identification of human endogenous retroviruses with complex mRNA expression and particle formation. Proc Natl Acad Sci U S A. 1993 May 15;90(10):4480–4484. doi: 10.1073/pnas.90.10.4480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariette X., Agbalika F., Daniel M. T., Bisson M., Lagrange P., Brouet J. C., Morinet F. Detection of human T lymphotropic virus type I tax gene in salivary gland epithelium from two patients with Sjögren's syndrome. Arthritis Rheum. 1993 Oct;36(10):1423–1428. doi: 10.1002/art.1780361015. [DOI] [PubMed] [Google Scholar]
- Maul G. G., Jimenez S. A., Riggs E., Ziemnicka-Kotula D. Determination of an epitope of the diffuse systemic sclerosis marker antigen DNA topoisomerase I: sequence similarity with retroviral p30gag protein suggests a possible cause for autoimmunity in systemic sclerosis. Proc Natl Acad Sci U S A. 1989 Nov;86(21):8492–8496. doi: 10.1073/pnas.86.21.8492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myskowski P. L., Straus D. J., Safai B. Lymphoma and other HIV-associated malignancies. J Am Acad Dermatol. 1990 Jun;22(6 Pt 2):1253–1260. doi: 10.1016/0190-9622(90)70171-d. [DOI] [PubMed] [Google Scholar]
- Nagasaki M., Morikawa S., Harada T., Miyasaka N. A novel EBV-related nucleo-cytoplasmic antigen in a null cell-line (HLN-STL-C) reactive to antibodies in the sera from patients with Sjögren's syndrome. J Autoimmun. 1989 Aug;2(4):457–462. doi: 10.1016/0896-8411(89)90175-3. [DOI] [PubMed] [Google Scholar]
- Olive D. M., Simsek M., Al-Mufti S. Polymerase chain reaction assay for detection of human cytomegalovirus. J Clin Microbiol. 1989 Jun;27(6):1238–1242. doi: 10.1128/jcm.27.6.1238-1242.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palomaki G. E., Knight G. J., Kloza E. M., Haddow J. E. Maternal weight adjustment and low serum alpha-fetoprotein values. Lancet. 1985 Feb 23;1(8426):468–468. doi: 10.1016/s0140-6736(85)91197-3. [DOI] [PubMed] [Google Scholar]
- Papadopoulos G. K., Moutsopoulos H. M. Slow viruses and the immune system in the pathogenesis of local tissue damage in Sjögren's syndrome. Ann Rheum Dis. 1992 Jan;51(1):136–138. doi: 10.1136/ard.51.1.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pflugfelder S. C., Wilhelmus K. R., Osato M. S., Matoba A. Y., Font R. L. The autoimmune nature of aqueous tear deficiency. Ophthalmology. 1986 Dec;93(12):1513–1517. doi: 10.1016/s0161-6420(86)33528-0. [DOI] [PubMed] [Google Scholar]
- Povolotsky J., Gold J. W., Chein N., Baron P., Armstrong D. Differences in human immunodeficiency virus type 1 (HIV-1) anti-p24 reactivities in serum of HIV-1-infected and uninfected subjects: analysis of indeterminate western blot reactions. J Infect Dis. 1991 Feb;163(2):247–251. doi: 10.1093/infdis/163.2.247. [DOI] [PubMed] [Google Scholar]
- Query C. C., Keene J. D. A human autoimmune protein associated with U1 RNA contains a region of homology that is cross-reactive with retroviral p30gag antigen. Cell. 1987 Oct 23;51(2):211–220. doi: 10.1016/0092-8674(87)90148-6. [DOI] [PubMed] [Google Scholar]
- Ranki A., Johansson E., Krohn K. Interpretation of antibodies reacting solely with human retroviral core proteins. N Engl J Med. 1988 Feb 18;318(7):448–449. doi: 10.1056/NEJM198802183180712. [DOI] [PubMed] [Google Scholar]
- Reddy M. M., Vodian M., Grieco M. H. Elevated levels of CD4 antigen in sera of human immunodeficiency virus-infected populations. J Clin Microbiol. 1990 Aug;28(8):1744–1746. doi: 10.1128/jcm.28.8.1744-1746.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rey M. A., Spire B., Dormont D., Barre-Sinoussi F., Montagnier L., Chermann J. C. Characterization of the RNA dependent DNA polymerase of a new human T-lymphotropic retrovirus (lymphadenopathy associated virus). Biochem Biophys Res Commun. 1984 May 31;121(1):126–133. doi: 10.1016/0006-291x(84)90696-x. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Bugawan T. L., Horn G. T., Mullis K. B., Erlich H. A. Analysis of enzymatically amplified beta-globin and HLA-DQ alpha DNA with allele-specific oligonucleotide probes. Nature. 1986 Nov 13;324(6093):163–166. doi: 10.1038/324163a0. [DOI] [PubMed] [Google Scholar]
- Saito I., Servenius B., Compton T., Fox R. I. Detection of Epstein-Barr virus DNA by polymerase chain reaction in blood and tissue biopsies from patients with Sjogren's syndrome. J Exp Med. 1989 Jun 1;169(6):2191–2198. doi: 10.1084/jem.169.6.2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai H., Kawamura M., Sakuragi J., Sakuragi S., Shibata R., Ishimoto A., Ono N., Ueda S., Adachi A. Integration is essential for efficient gene expression of human immunodeficiency virus type 1. J Virol. 1993 Mar;67(3):1169–1174. doi: 10.1128/jvi.67.3.1169-1174.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai H., Shibata R., Sakuragi J., Sakuragi S., Kawamura M., Adachi A. Cell-dependent requirement of human immunodeficiency virus type 1 Vif protein for maturation of virus particles. J Virol. 1993 Mar;67(3):1663–1666. doi: 10.1128/jvi.67.3.1663-1666.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarngadharan M. G., Robert-Guroff M., Gallo R. C. DNA polymerases of normal and neoplastic mammalian cells. Biochim Biophys Acta. 1978 Dec 11;516(4):419–487. doi: 10.1016/0304-419x(78)90019-7. [DOI] [PubMed] [Google Scholar]
- Sawada S., Sugai S., Iijima S., Takei M., Paredes E., Hayama T., Nishinarita S., Hosokawa Y., Horie T., Obara T. Increased soluble CD4 and decreased soluble CD8 molecules in patients with Sjögren's syndrome. Am J Med. 1992 Feb;92(2):134–140. doi: 10.1016/0002-9343(92)90103-i. [DOI] [PubMed] [Google Scholar]
- Schiødt M., Greenspan D., Daniels T. E., Nelson J., Leggott P. J., Wara D. W., Greenspan J. S. Parotid gland enlargement and xerostomia associated with labial sialadenitis in HIV-infected patients. J Autoimmun. 1989 Aug;2(4):415–425. doi: 10.1016/0896-8411(89)90170-4. [DOI] [PubMed] [Google Scholar]
- Schiødt M., Greenspan D., Levy J. A., Nelson J. A., Chernoff D., Hollander H., Greenspan J. S. Does HIV cause salivary gland disease? AIDS. 1989 Dec;3(12):819–822. doi: 10.1097/00002030-198912000-00006. [DOI] [PubMed] [Google Scholar]
- Seiki M., Hattori S., Hirayama Y., Yoshida M. Human adult T-cell leukemia virus: complete nucleotide sequence of the provirus genome integrated in leukemia cell DNA. Proc Natl Acad Sci U S A. 1983 Jun;80(12):3618–3622. doi: 10.1073/pnas.80.12.3618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shattles W. G., Brookes S. M., Venables P. J., Clark D. A., Maini R. N. Expression of antigen reactive with a monoclonal antibody to HTLV-1 P19 in salivary glands in Sjögren's syndrome. Clin Exp Immunol. 1992 Jul;89(1):46–51. doi: 10.1111/j.1365-2249.1992.tb06875.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strand V., Talal N. Advances in the diagnosis and concept of Sjögren's syndrome (autoimmune exocrinopathy). Bull Rheum Dis. 1979;30(9):1046–1052. [PubMed] [Google Scholar]
- Sölder B., Marschang P., Wachter H., Dierich M. P., Nayyar S., Lewin I. V., Stanworth D. R. Anti-viral antibodies in HIV (HTLV-III) infection possess auto-antibody activity against a CH1 domain determinant in human IgG: possible immunological consequences. Immunol Lett. 1989 Nov;23(1):9–19. doi: 10.1016/0165-2478(89)90148-x. [DOI] [PubMed] [Google Scholar]
- Talal N., Dauphinée M. J., Dang H., Alexander S. S., Hart D. J., Garry R. F. Detection of serum antibodies to retroviral proteins in patients with primary Sjögren's syndrome (autoimmune exocrinopathy). Arthritis Rheum. 1990 Jun;33(6):774–781. doi: 10.1002/art.1780330603. [DOI] [PubMed] [Google Scholar]
- Tseng C. C., Nio Y., Shiraishi T., Tsubono M., Morimoto H., Kawabata K., Masai Y., Tun T., Fukumoto M., Tobe T. Comparative study on various combination chemotherapies against human gastric cancer xenograft lines of well- and poorly-differentiated adenocarcinomas transplanted in nude mice. Anticancer Drugs. 1991 Oct;2(5):457–464. doi: 10.1097/00001813-199110000-00004. [DOI] [PubMed] [Google Scholar]
- Ulirsch R. C., Jaffe E. S. Sjögren's syndrome-like illness associated with the acquired immunodeficiency syndrome-related complex. Hum Pathol. 1987 Oct;18(10):1063–1068. doi: 10.1016/s0046-8177(87)80223-x. [DOI] [PubMed] [Google Scholar]
- Vernant J. C., Buisson G., Magdeleine J., De Thore J., Jouannelle A., Neisson-Vernant C., Monplaisir N. T-lymphocyte alveolitis, tropical spastic paresis, and Sjögren syndrome. Lancet. 1988 Jan 23;1(8578):177–177. doi: 10.1016/s0140-6736(88)92744-4. [DOI] [PubMed] [Google Scholar]
- Wain-Hobson S., Sonigo P., Danos O., Cole S., Alizon M. Nucleotide sequence of the AIDS virus, LAV. Cell. 1985 Jan;40(1):9–17. doi: 10.1016/0092-8674(85)90303-4. [DOI] [PubMed] [Google Scholar]
- Waite M. R., Allen P. T. RNA-directed DNA polymerase activity of reticuloendotheliosis virus: characterization of the endogenous and exogenous reactions. J Virol. 1975 Oct;16(4):872–879. doi: 10.1128/jvi.16.4.872-879.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warnke R., Levy R. Detection of T and B cell antigens hybridoma monoclonal antibodies: a biotin-avidin-horseradish peroxidase method. J Histochem Cytochem. 1980 Aug;28(8):771–776. doi: 10.1177/28.8.7003003. [DOI] [PubMed] [Google Scholar]
- Whaley K., Williamson J., Chisholm D. M., Webb J., Mason D. K., Buchanan W. W. Sjogren's syndrome. I. Sicca components. Q J Med. 1973 Apr;42(166):279–304. [PubMed] [Google Scholar]
- Wiley C. A., Nerenberg M., Cros D., Soto-Aguilar M. C. HTLV-I polymyositis in a patient also infected with the human immunodeficiency virus. N Engl J Med. 1989 Apr 13;320(15):992–995. doi: 10.1056/NEJM198904133201507. [DOI] [PubMed] [Google Scholar]
- de Clerck L. S., Couttenye M. M., de Broe M. E., Stevens W. J. Acquired immunodeficiency syndrome mimicking Sjögren's syndrome and systemic lupus erythematosus. Arthritis Rheum. 1988 Feb;31(2):272–275. doi: 10.1002/art.1780310216. [DOI] [PubMed] [Google Scholar]