FIGURE 2.
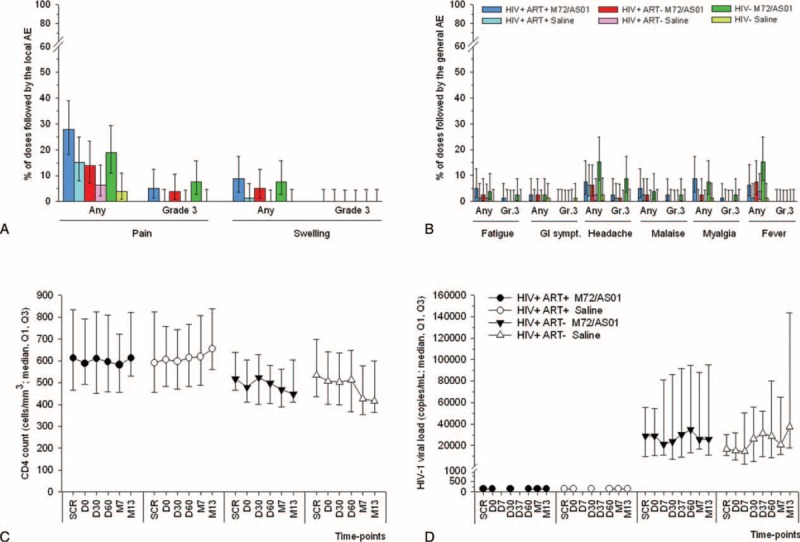
Safety and reactogenicity including HIV-specific parameters. Percentage of doses with 95% CI followed by solicited local (A) and general (B) AEs reported within 7 days postvaccination are shown for all subjects for whom at least 1 administration of vaccine or control was documented (the Total Vaccinated Cohort). GI sympt.: gastro-intestinal symptoms. CD4+ T-cell counts (C) and HIV-1 viral loads (D) obtained from the Total Vaccinated Cohort were measured at screening (SCR), prior to each vaccination (D0 and D30), and 1, 6, or 12 months after the 2nd dose (D60, M7, M13). In addition, HIV viral loads were measured at 7 days post each dose (D7 and D37). The detection limit of the HIV-1 viral load assay was 40 copies/mL. AE = adverse event, CI = confidence interval, HIV = human immunodeficiency virus.
